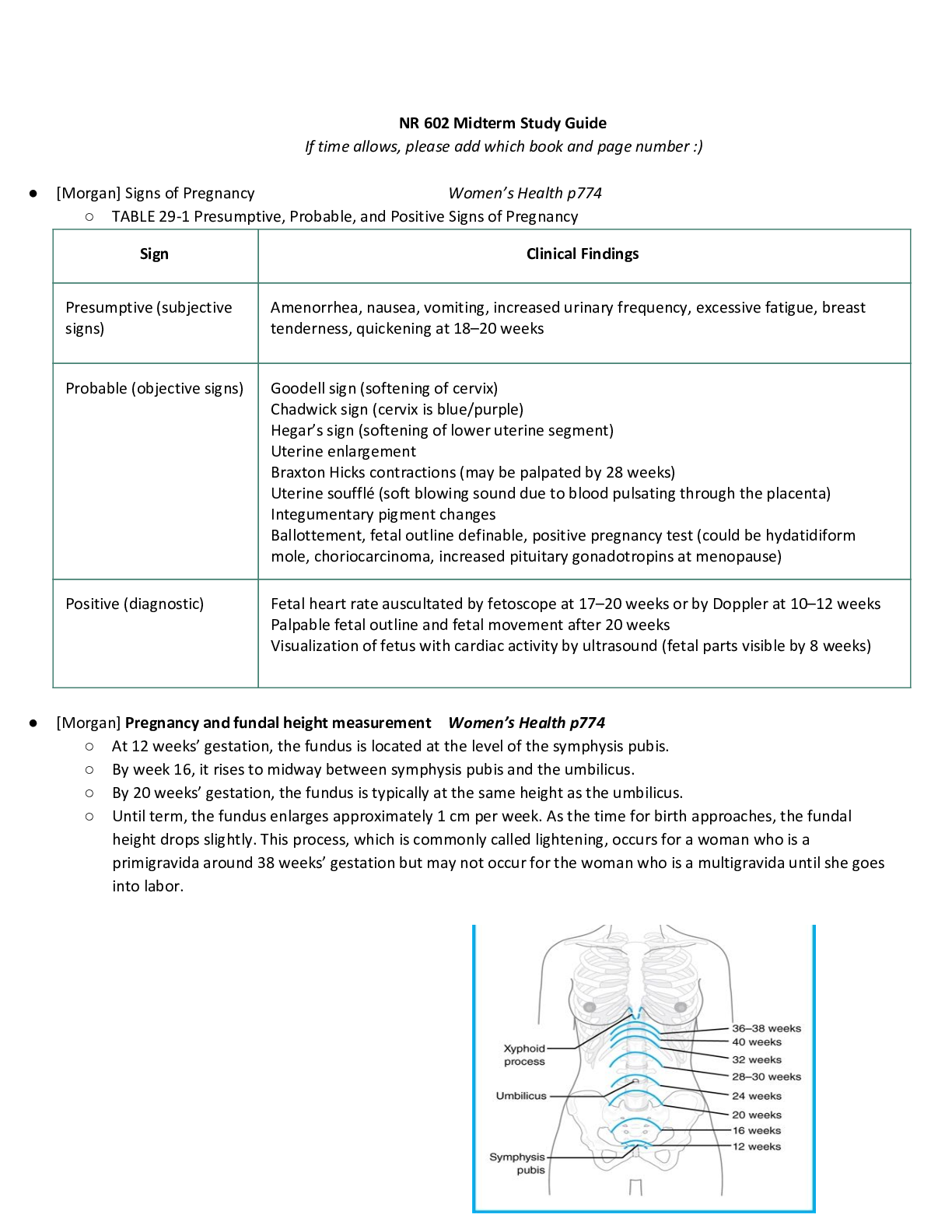
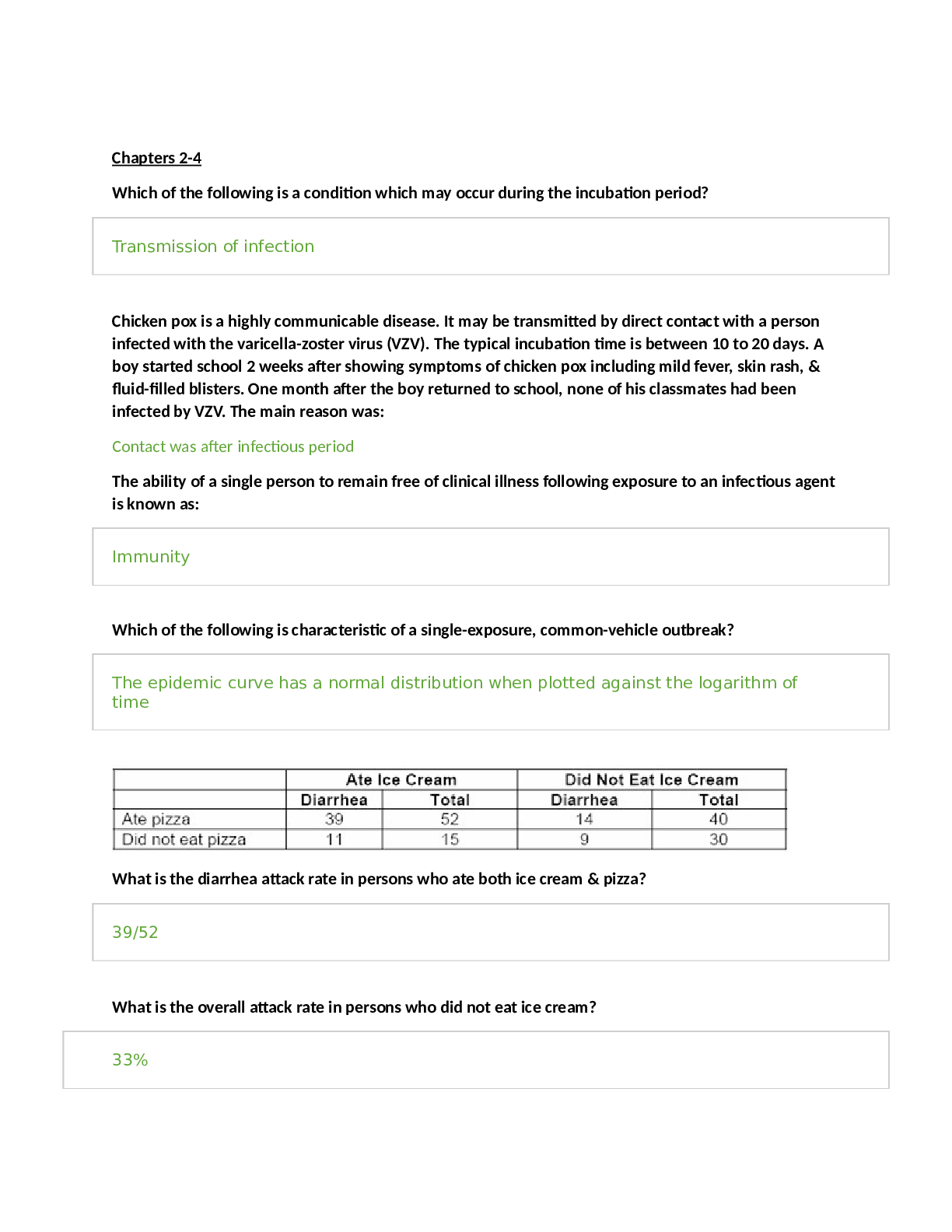
Health Care > STUDY GUIDE > CITI TRAINING EXAM STUDY GUIDE | 390 Questions with 100% Correct Answers | 39 Pages (All)
CITI TRAINING EXAM STUDY GUIDE | 390 Questions with 100% Correct Answers | 39 Pages
Document Content and Description Below
An example of an institutional COI is: - ✔✔An industry sponsor pays for the construction of a new research laboratory at the organization The peer review process can create conflicts of interest... because the choice of who reviews a potentially publishable project may show: - ✔✔There may be bias by the peer reviewer as to the area of research During an Institutional Review Board (IRB) meeting, any IRB member who may have a potential COI with a study under review should: - ✔✔Disclose their potential COI and may answer questions, but recuse themselves from voting A researcher's membership on an advisory board with an organization sponsoring research can create a COI because: - ✔✔It may be difficult for the researcher to appear neutral, as the researcher may have an interest in the research's success The FDA regulations governing disclosure of individual COIs require: - ✔✔Applicants submitting marketing applications to disclose financial COIs of researchers who conducted clinical studies An example of an individual financial COI is: - ✔✔A researcher's spouse holds equity in a publicly traded pharmaceutical company that is also the sponsor of the researcher's study. A researcher calls you stating that he plans to submit a proposal to the NIH for a human subjects research study. He wants to know at what point he and his study team must submit COI disclosures to comply with the PHS regulation. - ✔✔No later than the time of proposal submission For clinical trials, what should be reported to the Office for Clinical Research (OCR) to confirm ClinicalTrials.gov registration and for the purpose of ensuring Medicare reimbursement? - ✔✔NCT number The Sunshine Act requires manufacturers to report "payments of transfers of value" for how much annually? - ✔✔$10.00 Which department reviews a sample of clinical trials being conducted in each department and provides education, tools, and corrective and preventative action plans, when needed? - ✔✔Clinical Trials Audit and Compliance Getting your proposal prepared for review and approval can be cumbersome. Which department in the Office of Research Administration assists with proposal preparation? - ✔✔Research Administration Services (RAS) [Show More]
Last updated: 1 year ago
Preview 1 out of 39 pages

Also available in bundle (1)

Bundle for CITI Certification Tests Compilation
Bundle for CITI Certification Tests Compilation
By Tessa 1 year ago
$29
8
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 12, 2022
Number of pages
39
Written in
Additional information
This document has been written for:
Uploaded
Aug 12, 2022
Downloads
0
Views
58

















 Rasmussen College.png)