*NURSING > QUESTIONS & ANSWERS > Miami Dade College, Miami NUR 1234: Lesson 06 - Acid-Base Imbalance_ NRSG2570_ Multisystem Disorders (All)
Miami Dade College, Miami NUR 1234: Lesson 06 - Acid-Base Imbalance_ NRSG2570_ Multisystem Disorders. All Correct Answers Indicated. LATEST FOR 2021/2022
Document Content and Description Below
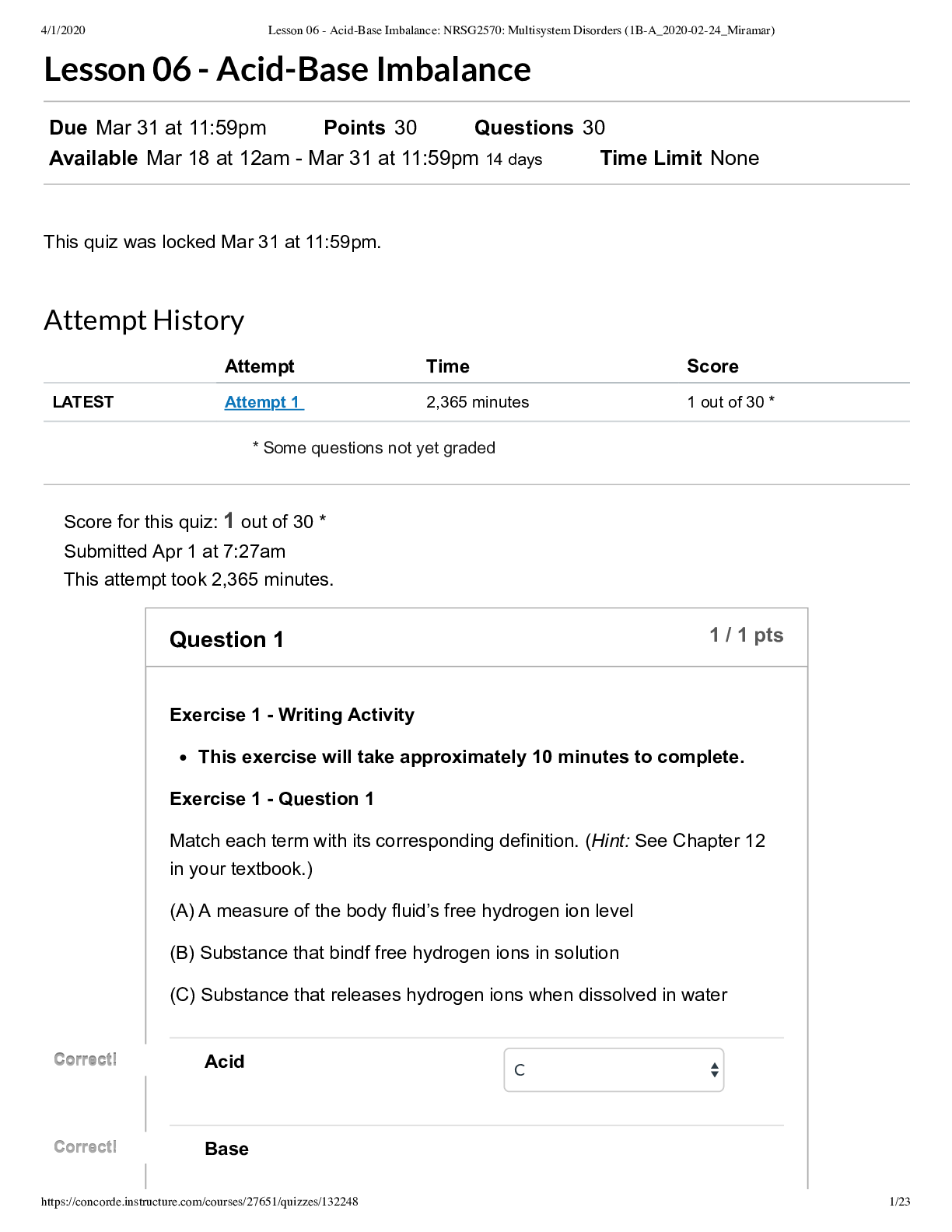
-24_Miramar) /132248 1/23 * Some questions not yet graded Lesson 06 - Acid-Base Imbalance Due Mar 31 at 11:59pm Points 30 Questions 30 Available Mar 18 at 12am - Mar 31 at 11:59pm 14 days Time Limit N... one This quiz was locked Mar 31 at 11:59pm. Attempt History Attempt Time Score LATEST Attempt 1 2,365 minutes 1 out of 30 * Score for this quiz: 1 out of 30 * Submitted Apr 1 at 7:27am This attempt took 2,365 minutes. Question 1 1 / 1 pts Exercise 1 - Writing Activity This exercise will take approximately 10 minutes to complete. Exercise 1 - Question 1 Match each term with its corresponding definition. (Hint: See Chapter 12 in your textbook.) (A) A measure of the body fluid’s free hydrogen ion level (B) Substance that bindf free hydrogen ions in solution (C) Substance that releases hydrogen ions when dissolved in water Acid C Base Correct! Correct! Correct! Correct! -24_Miramar) /132248 2/23 B Correct! Correct! pH A Question 2 Not yet graded / 1 pts Exercise 1 - Question 2 Identify and describe three methods of acid-basis homeostasis. Indicate the type of defense and the associated mechanisms of action. First line of defense Second line of defense Third line of defense 1. Buffers -First line of defense against pH changes in body fluids -Chemicals that help control pH of body fluids -Contained in all body fluids -Consist of weak acids that reversibly take up hydrogen ions when a fluid is too acidic Types: Bicarbonate buffers Phosphate buffers Hemoglobin buffers Protein buffers 2. Repiratory System/Contribution Second defense against acid-base disorders Lungs excrete CO2 and water from the body Changes in rate and depth of respiration Result from stimulation of chemoreceptors that sense PaCo2 and pH blood -24_Miramar) /132248 3/23 Exter an influence on and sere to alter amount of PaCO2 in blood 3. Renal Contribution: -Third defense against acid-base disorders -Can excrete any acid from the body except carbonicacid (solely excreted by the lungs) -Kidney contributes in acid-base balance by: 1.Excreting HCO3 2.Secreting H 3. Producing NH4 -Renal compensatory response to an imbalance of carbonic acid requires several days to be fully operative -24_Miramar) /132248 4/23 First line of defense Type of defense: Chemical/protein buffers Mechanisms of action: Chemicals (bicarbonate and phosphate) and/or proteins (albumin, globulin, and hemoglobin [Hgb]) rapidly respond to changes in pH by acting as hydrogen ion “sponges.” They can bind hydrogen ions when too many are present or release hydrogen ions when not enough are present. Second line of defense Type of defense: Respiratory Mechanisms of action: Works when chemical buffers alone cannot maintain homeostasis. Special receptors in the respiratory areas of the brain respond to changes in amount of carbon dioxide (CO ) in brain tissues. As the amount increases, the brain triggers an increase in the rate and depth of respirations to blow off the CO . As the amount decreases, central receptors slow the neurons to decrease the rate and depth of respirations, thus increasing CO retention. Third line of defense Type of defense: Renal Mechanisms of action: Stronger than the first two lines of defense, but takes longer to respond (24-48 hours). When blood pH changes are persistent, renal mechanisms increase excretion and reabsorption rates of acids or bases dependent on direction of the pH changes. Involves three mechanisms: (1) kidney movement of bicarbonate (when blood hydrogen ion levels are high, this bicarbonate is reabsorbed from the kidneys back into circulation, where it can help buffer excess hydrogen ions; when blood hydrogen ion levels are low, the bicarbonate remains in the urine and is excreted); (2) formation of acids (phosphate in urine draws and binds to hydrogen, which is then excreted); and (3) formation of ammonium (ammonia secreted into the urine combines with hydrogen to form ammonium, which is then excreted). 2 2 2 -24_Miramar) /132248 5/23 Question 3 Not yet graded / 1 pts Exercise 2 - Virtual Hospital Activity This exercise will take approximately 45 minutes to complete. Sign in to work at Pacific View Regional Hospital for Period of Care 1. (Note: If you are already in the virtual hospital from a previous exercise, click on Leave the Floor and then on Restart the Program to get to the sign-in window.) From the Patient List, select Jacquline Catanazaro (Room 402). Click on Go to Nurses’ Station. Click on Chart and then on 402. Click on History and Physical. Exercise 2 - Question 1 Is there anything in Jacquline Catanazaro’s history that would put her at risk for an acid-base imbalance? History of asthma; has not been taking the medication as prescribed Question 4 Not yet graded / 1 pts Click on Return to Nurses’ Station. Click on 402 at the bottom of your screen. Click on Patient Care and then on Nurse-Client Interactions. Select and view the video titled 0730: Intervention—Airway. (Note: Check the virtual clock to see whether enough time has elapsed. You can use the fast-forward feature to advance the time by 2-minute intervals if the video is not yet available. Then click again on Patient Care and Nurse-Client Interactions to refresh the screen.) Exercise 2 - Question 2 -24_Miramar) /132248 6/23 Based on Jacquline Catanazaro’s history, what would the nurse expect to be causing her respiratory distress? Asthma exacerbation Question 5 Not yet graded / 1 pts Exercise 2 - Question 3 Why is the nurse waiting until after the arterial blood gases (ABGs) are drawn to give the patient a nebulizer treatment? To get a true picture of Jacquline Catanazaro’s acid-base balance while the patient is exhibiting respiratory distress Question 6 Not yet graded / 1 pts Click on Chart and then on 402. Click on Laboratory Reports. Exercise 2 - Question 4 Document the results of Jacquline Catanazaro’s ABG on Monday at 1030. pH PaO PaCO O sat 2 2 2 -24_Miramar) /132248 7/23 Bicarb pH: 7.33 PaO : 80 PaCO : 48 O sat: 85% Bicarb: 23 2 2 2 Question 7 Not yet graded / 1 pts Exercise 2 - Question 5 Document the results of Jacquline Catanazaro’s ABG on Wednesday at 0730. pH PaO PaCO O sat Bicarb 2 2 2 -24_Miramar) /132248 8/23 pH: 7.35 PaO : 80 PaCO : 50 O sat: 85% Bicarb: 24 2 2 2 Question 8 Not yet graded / 1 pts Exercise 2 - Question 6 How would you interpret the results documented in questions 4 and 5? Is the acid-base imbalance compensated or uncompensated (fully or partially)? Explain your answer. Monday: Partially compensated respiratory acidosis. The pH is lower than normal (7.35-7.45), denoting acidosis. The PaCO is slightly above normal (35-45), also denoting acidosis. The bicarb is within normal limits (21-28); it takes 24-48 hours for the renal system to begin to compensate for acidosis. Wednesday: Fully compensated respiratory acidosis. The pH is at the lower limit of normal, but the PaCO is increased with the bicarb remaining within normal limits. It is fully compensated because the pH is within normal limits. 2 2 Question 9 Not yet graded / 1 pts -24_Miramar) /132248 9/23 Exercise 2 - Question 7 Based on the acute aspect of Jacquline Catanazaro’s respiratory difficulties, what lines of defense would you expect to be working to compensate for her respiratory acidosis? Because this is an acute respiratory problem, the chemical/protein buffer system (first line of defense) should be responding to bind to the extra hydrogen ions and increase the serum pH. The third line of defense, the renal system, will take 24-48 hours to respond to and begin compensation for the acidosis. Question 10 Not yet graded / 1 pts Exercise 2 - Question 8 If the patient’s electrolyte results were available, what might you expect her potassium levels to be? Provide a rationale for your answer. (Hint: See Chapter 12 in your textbook.) The potassium may be elevated. In acidosis, the extracellular hydrogen ion content increases, and the hydrogen ions move into the intracellular fluid. To keep the intracellular fluid electrically neutral, an equal number of potassium ions leave the cell, creating a relative hyperkalemia. If the acidosis is chronic, the kidneys have time to compensate for the excess hydrogen and hyperkalemia does not occur. -24_Miramar) /132248 10/23 Question 11 Not yet graded / 1 pts Exercise 2 - Question 9 Based on Jacquline Catanazaro’s medical diagnosis, what is the underlying pathophysiologic problem leading to the respiratory acidosis? (Hint: See Chapter 12 in your textbook.) Airway obstruction—the asthma is causing bronchoconstriction and therefore decreased air movement in and out of the lungs. This decreases gas exchange and causes CO retention as well as hypoxia. 2 Question 12 Not yet graded / 1 pts Click on Return to Room 402. Click on Patient Care and then Physical Assessment. Complete a physical assessment for Jacquline Catanazaro by clicking on the body system categories (yellow buttons) and subcategories (green buttons). Exercise 2 - Question 10 Record the findings of your physical assessment below. Mental status Musculoskeletal Cardiovascular Respiratory Integumentary -24_Miramar) /132248 11/23 Mental status: Alert and oriented x 3. Perceptual ability slightly impaired. Agitated and anxious. Musculoskeletal: Full range of movement. Moves all extremities equally. Reflexes intact. Cardiovascular: Apical pulse regular. No JVD. S and S normal. Respiratory: Labored and shallow respiratory effort. Tachypneic. Crackles in right lower lobes, wheezes throughout. Productive cough with white frothy sputum. Substernal retractions and use of neck muscles. Integumentary: Skin warm and moist with flushed color. 1 2 Question 13 Not yet graded / 1 pts Exercise 2 - Question 11 Are there any clinical manifestations of respiratory acidosis? If so, please describe. If not, how do you explain? None of the identified key features of acidosis are identified in this patient. The patient is no longer acidotic at the time of this assessment. Question 14 Not yet graded / 1 pts Click on Take Vital Signs. -24_Miramar) /132248 12/23 Exercise 2 - Question 12 What is the patient’s respiratory rate? How does this correlate with her respiratory acidosis? The exact rate will vary depending on the actual time assessed, but the respiratory rate is elevated in attempt to blow off excess CO2 and normalize pH. Question 15 Not yet graded / 1 pts Exercise 2 - Question 13 If Jacquline Catanazaro’s pH were 7.2, how might her physical assessment differ? Document the expected clinical manifestations of respiratory acidosis below. Neurologic Musculoskeletal Cardiovascular Respiratory Integumentary -24_Miramar) /132248 13/23 Neurologic: Lethargy, confusion, stupor, coma Musculoskeletal: Hyporeflexia, skeletal muscle weakness, flaccid paralysis Cardiovascular: Bradycardia, tall, peaked T waves; widened QRS complexes, prolonged PR interval; heart blocks, hypotension, thready peripheral pulses Respiratory: Variable, ineffective respirations Integumentary: Pale to cyanotic mucous membranes Question 16 Not yet graded / 1 pts Click on Chart. Click on 402 for Jacquline Catanazaro’s chart. Click on Physician’s Orders. Exercise 2 - Question 14 Look at the most recent physician’s orders. What medication is ordered to treat the respiratory acidosis? What is the medication’s underlying mechanism of action to correct the acidosis? (Hint: See Drug Guide.) Albuterol 5 mg via nebulizer x 1 STAT—this is a bronchodilator, which will relieve the airway obstruction caused by the asthma. Dilating the bronchi will increase air movement and gas exchange at the alveolar level, thus increasing CO excretion and raising the patient’s pH to normal. 2 -24_Miramar) /132248 14/23 Question 17 Not yet graded / 1 pts Click on Return to Room 402. Click on Leave the Floor. Click on Restart the Program. Sign in to work at Pacific View Regional Hospital for Period of Care 2. From the Patient List, select Jacquline Catanazaro (Room 402). Click on Go to Nurses’ Station. Click on Chart and then on 402. Click on Laboratory Reports. Scroll down to review the results for Wed 1000. Exercise 2 - Question 15 Look at the ABGs drawn at 1000. Interpret the ABGs. Was the treatment effective? The ABGs are now normal with a pH of 7.40 and a PaCO of 40. The medication was effective in increasing air movement and gas exchange. Oxygenation is also improved as demonstrated by an increase in the PaO and the oxygen saturation. 2 2 Question 18 Not yet graded / 1 pts Exercise 3 - Virtual Hospital Activity This exercise will take approximately 45 minutes to complete. Sign in to work at Pacific View Regional Hospital for Period of Care 1. (Note: If you are already in the virtual hospital from a previous exercise, click on Leave the Floor and then on Restart the Program to get to the sign-in window.) From the Patient List, select Patricia Newman (Room 406). Click on Go to Nurses’ Station. Click on Chart and then on 406. -24_Miramar) /132248 15/23 Click on History and Physical. Exercise 3 - Question 1 Is there anything in Patricia Newman’s history that would put her at risk for an acid-base imbalance? Emphysema for 12 years Smoker Probable pneumonia this admission Question 19 Not yet graded / 1 pts Click on Laboratory Reports. Exercise 3 - Question 2 Document the results of Patricia Newman's two most recent ABGs (Tuesday at 2300 and Wednesday at 0500) below. Include pH, PaO , PaCO , O sat, and bicarb. Tuesday 2300 Wednesday 0500 2 2 2 -24_Miramar) /132248 16/23 Tuesday 2300 pH: 7.35 PaO : 72 PaCO : 45 O sat: 93% Bicarb: 25 Wednesday 0500 pH: 7.33 PaO : 70 PaCO : 47 O sat: 92% Bicarb: 26 2 2 2 2 2 2 Question 20 Not yet graded / 1 pts Exercise 3 - Question 3 How would you interpret the results you recorded in the previous table? Is the acid-base imbalance compensated or uncompensated (fully or partially)? Explain your answer. -24_Miramar) /132248 17/23 Tuesday: Normal acid-base balance with hypoxemia. pH is at the lower limit (towards the acidotic side), PaO is decreased (normal is 80-100). PaCO is at the upper limit of normal (towards the acidotic side), as is the bicarbonate level. Wednesday: Partially compensated respiratory acidosis. The pH is decreased (acidotic), and the PaCO is elevated (acidotic as well). Although the bicarbonate level is within normal limits (22-28), it is slightly elevated from last evening and demonstrates a partial compensation to maintain pH near normal. 2 2 2 Question 21 Not yet graded / 1 pts Exercise 3 - Question 4 Based on the chronic aspect of Patricia Newman’s respiratory difficulties, what lines of defense would you expect to be working to compensate for the respiratory acidosis? Because this is a chronic respiratory problem, the first and third lines of defense will be working to maintain the pH in a normal or near normal range. The chemical/protein buffers will respond to acute changes but can only neutralize a finite amount of hydrogen ions. Because Patricia Newman’s emphysema is a chronic condition, the kidneys respond to the elevated PaCO levels by excreting more hydrogen ions and resorbing more bicarbonate. In Patricia Newman’s case, although the PaCO and bicarbonate levels are within normal limits on Tuesday, the renal system is compensating somewhat for the higher PaCO level by keeping the bicarbonate levels equally high. As the pH drops slightly on Wednesday, the kidneys work to retain more bicarbonate. 2 2 2 -24_Miramar) /132248 18/23 Question 22 Not yet graded / 1 pts Exercise 3 - Question 5 Based on the ABG results, has Patricia Newman’s condition improved or worsened since admission the evening before? Worsened Question 23 Not yet graded / 1 pts Click on Nurse's Notes. Exercise 3 - Question 6 Read the notes for Wednesday 0730. Describe the actions taken by the nurse. Are they appropriate or not? Explain your answer. The nurse reapplied the patient’s nasal cannula after assessing lung sounds and ABG results. These are appropriate actions as long as there was not a significant time delay in applying the oxygen while waiting to review the ABG results. The pulse oximetry itself demonstrated hypoxemia and the need for supplemental O for the patient. The ABG results verified the hypoxemia and resultant increase in PaCO . 2 2 -24_Miramar) /132248 19/23 Question 24 Not yet graded / 1 pts Exercise 3 - Question 7 What additional actions do you think would be appropriate at this time? Patient teaching regarding the need to keep O cannula in place. Notify physician of ABG results because the patient is becoming slightly acidotic—the administration of O itself may not correct this. 2 2 Question 25 0 / 1 pts Click on Laboratory Results. Exercise 3 - Question 8 The patient's serum potassium level is ______ mEq/L. 3.2 ou Answered ou Answered orrect Answers orrect Answers Question 26 Not yet graded / 1 pts Click on Physician's Orders. Exercise 3 - Question 9 -24_Miramar) /132248 20/23 How would you explain the patient’s hypokalemia in relation to respiratory acidosis? (Hint: See the Drug Guide and Chapter 12 in your textbook.) Potassium levels are elevated in patients with acute respiratory acidosis but are normal in patients with chronic respiratory acidosis secondary to renal compensation. With acute acidosis, potassium is exchanged for hydrogen in the blood to decrease pH and maintain electroneutrality. This is not necessary with renal compensation because the excess hydrogen ions are excreted. Also, Patricia Newman is on chlorothiazide, a diuretic that causes potassium excretion; this is the reason for the hypokalemia. Question 27 Not yet graded / 1 pts Exercise 3 - Question 10 Based on Patricia Newman’s medical diagnosis, what is the underlying pathophysiologic problem leading to the respiratory acidosis? How does that differ from Jacquline Catanazaro’s problem in the previous exercise of this lesson? (Hint: See Chapter 12 in your textbook.) -24_Miramar) /132248 21/23 Patricia Newman’s problem is twofold: Reduced alveolar-capillary diffusion: The pathophysiologic changes in the alveoli of a patient with pneumonia impair gas exchange at the alveolar-capillary membranes, causing carbon dioxide retention and acidosis. Airway obstruction: The pathophysiologic changes of emphysema at the alveolar level prevent adequate air movement, which leads to poor gas exchange as well, particularly causing air-trapping and CO retention. Both Jacquline Catanazaro and Patricia Newman have airway obstruction. However, Jacquline Catanazaro’s major problem is getting air movement through constricted bronchials, whereas Patricia Newman’s problem is exhaling air outward. Additionally, the pneumonia is causing reduced diffusion in Patricia Newman, worsening her gas exchange. 2 Question 28 Not yet graded / 1 pts Click on Return to Nurses' Station. Click on 406 at the bottom of your screen. Click on Patient Care and then on Physical Assessment. Complete a physical assessment for Patricia Newman by clicking on the body categories (yellow buttons) and subcategories (green buttons). Click on Take Vital Signs. Exercise 3 - Question 11 Record the findings of your physical assessment below. Mental status Musculoskeletal Cardiovascular Respiratory Integumentary Mental status: Awake, alert, and oriented; mild anxiety related to respiratory difficulty Musculoskeletal: Mild weakness due to fatigue, reflexes intact Cardiovascular: Sinus tachycardia; S1 and S2 WNL; no clicks, rubs, or murmurs; +2 peripheral pulses Respiratory: Labored and shallow respirations; rate 31 (answers may vary depending on the actual time assessed); using some accessory muscles; course crackles throughout; expectorating thick yellow sputum Integumentary: Skin warm, dry, and intact Question 29 Not yet graded / 1 pts Exercise 3 - Question 12 Does Patricia Newman demonstrate any clinical manifestations of respiratory acidosis? If so, please describe. If not, explain why not. No clinical manifestations of respiratory acidosis are evident. Patient is just mildly acidotic at 7.33. At this point, manifestations are at the cellular level. Question 30 Not yet graded / 1 pts -24_Miramar) /132248 23/23 Exercise 3 - Question 13 What nursing interventions could you, as a graduate nurse, plan and implement to improve Patricia Newman’s acid-base balance and prevent complications? [Show More]
Last updated: 1 year ago
Preview 1 out of 23 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 03, 2021
Number of pages
23
Written in
Additional information
This document has been written for:
Uploaded
Aug 03, 2021
Downloads
0
Views
46













 All Correct Answers, Download to Score A.png)





.png)

 Self-Assessment Examination (100 Questions With All Correct Answers) Complete solution.png)
.png)




 Med Surg test Latest Verified Questions and all Correct Answers with Explanations Chapter 57 Management of Patients with Female Reproducti.png)

