*NURSING > STUDY GUIDE > NR 602 WEEK 3 QUIZ Study Guide Completed A (All)
NR 602 WEEK 3 QUIZ Study Guide Completed A
Document Content and Description Below
NR 602 WEEK 3 QUIZ Upper Respiratory Tract Disorders The Common Cold The common cold is a frequent problem seen in pediatric practice, and parents often seek information from their child's primary... health care provider as to whether their child's symptoms represent a typical URI or indicate the beginning or advancing signs of a more serious illness. Young children have on average 6 to 10 URIs, or colds, per year. The typical course of these illnesses is an initial low-grade fever with clear rhinitis changing by day 3 to purulent discharge and to a slow resolution with clear nasal discharge by day 10 (Wald et al, 2013). Viruses cause most common colds, with 50% resulting from infection by the more than 100 serotypes of rhinoviruses. Parainfluenza viruses, respiratory syncytial virus (RSV), coronavirus, and human metapneumovirus are also common agents (Cherry and Nieves, 2009; Schuster and Williams, 2013). Other agents that occasionally cause cold symptoms include adenovirus, enterovirus, influenza viruses, reoviruses, and human bocavirus. Day care and preschool attendance are associated with an increased number of common colds in young children and their spread to school-age children in the family. The acquisition of a common cold virus occurs via inoculation of the nose and possibly the conjunctiva. Colds can be spread through direct inhalation of virus from a sneeze, nasal blowing, or inoculation via fingers from nasal secretions or fomites. Mental stress, lack of sleep, high basal levels of catecholamines, infrequent exercise, smoking, and low vitamin C intake are risk factors for colds in adults, but these have not been studied in children (Cherry and Nieves, 2009). Pathophysiology The viral infection of the nasopharyngeal mucosa initiates a host response that produces the symptoms of a cold. Once the cold viral is deposited on the nasal mucosa, it attaches to cell receptors and enters the cells. Potent cytokines including interleukin (IL)-8 attract large number of PMN cells. As a result, vascular permeability increases, causing the leak of plasma proteins into nasal secretions. Bradykinins cause the pharyngitis and rhinitis. The presence of PMN, rather than bacterial colonization, changes the color of nasal mucus, with yellow-green mucus due to PMN enzymatic activity and yellow mucus being caused by the simple presence of PMN. With rhinovirus, there is an increase in bradykinins and albumin in the nasal secretions but no increase in histamines. Rhinovirus and coronaviruses do not cause destruction of the nasal epithelium, but adenovirus and influenza have a significant destructive effect on the respiratory epithelium (Pappas and Hendley, 2011). Clinical Findings Symptoms of a viral cold include nasal congestion, cough, sneezing, rhinorrhea, fever, hoarseness, and pharyngitis (Fashner et al, 2012). Sleep disturbances do occur with URIs, but vomiting and diarrhea are uncommon. The symptoms should decrease at the end of 10 days. History The following may be reported: • Gradual onset • Prominent nasal symptoms of rhinorrhea (key finding) • Sore throat and dysphagia • Mild cough and poor sleep • Low-grade fever especially in younger children • After a variable period of 1 to 3 days, nasal secretions are thicker and more purulent, leading to nasal excoriation Physical Examination Virus-specific findings include: • Conjunctiva: Mild injection • Nose: Red nasal mucosa with secretions of varying colors depending on the degree of nasal mucosa destruction and PMN activity • Throat: Mild erythema • Lymph: Anterior cervical lymphadenopathy with freely movable nodes less than 2 cm • Chest: Clear to auscultation and without adventitious sounds Diagnostic StudiesA throat culture should not be done if there are nasal symptoms with complaints of throat pain. However, if the diagnosis of common cold is in doubt, a rapid antigen detection test (RADT), often called a rapid strep test, should be done with a throat culture if the RADT is negative (Shulman et al, 2012). Differential Diagnosis The most common differentials are allergic rhinitis, rhinosinusitis, and adenoiditis (Table 32-2). Management Only supportive care is needed for a viral URI. See the Basic Respiratory Management Strategies section and see Box 32-1 for guidance regarding the use of decongestants, antihistamines, and cough medications. Antibiotics are not appropriate treatment. The child should receive symptomatic relief for fever, pain, and nasal congestion using normal saline and an antipyretic. Although topically applied menthol may improve nighttime cough, there is a risk of chemical irritation and accidental ingestion, causing CNS and gastrointestinal (GI) side effects. Fluid intake should be encouraged. Complications Common colds are self-limiting, but secondary bacterial infections including otitis media, pneumonia, and sinusitis can occur along with secondary wheezing. Pharyngitis, Tonsillitis, and Tonsillopharyngitis Pharyngitis is an inflammation of the mucosa lining the structures of the throat, including the tonsils, pharynx, uvula, soft palate, and nasopharynx. It can be due to infectious agents or noninfectious causes, such as smoke or other air irritants. The illness is generally acute and involves an inflammatory response, including erythema, exudate, or ulceration. The etiology could include a number of viruses and bacteria. If there are nasal symptoms, it is called nasopharyngitis, but if there are no nasal symptoms, the disease is called pharyngitis or tonsillopharyngitis. Most cases of pharyngitis are caused by viruses (Gereige and Cunill-De Sautu, 2011). Adenovirus is the most common cause of nasopharyngitis (Cherry, 2009c). Other viruses include Epstein-Barr virus (EBV), herpes simplex virus (HSV), cytomegalovirus (CMV), enterovirus, influenza virus, parainfluenza, and human immunodeficiency virus (HIV). The viral organisms generally present with upper nasal symptoms. The common bacterial etiology is GABHS in children between 5 and 11 years old, whereas 40% of reported cases of gonococcal infections occur in females 15 to 19 years old. Other organisms include Corynebacterium diphtheriae, Arcanobacterium haemolyticum, Neisseria gonorrhoeae, group C and group G streptococci, Chlamydia trachomatis, Francisella tularensis, and Mycoplasma pneumonia (Gereige and Cunill-De Sautu, 2011). Acute Viral Pharyngitis, Tonsillitis, or Tonsillopharyngitis Adenoviruses are more likely to cause pharyngitis as a prominent symptom. Other viruses (e.g., rhinovirus) are associated with pharyngitis as a minor symptom and rhinorrhea or cough as predominant features. The enterovirus (coxsackievirus, echovirus), herpesvirus, and EBV are also common. Viral infections occur yearround, but adenovirus presenting as pharyngoconjunctival fever occurs in outbreaks during the summer due to contaminated swimming pools (Gereige and Cunill-De Sautu, 2011). It is helpful to know what agents are currently infecting children in the community. However, when a patient only has a sore throat, it is difficult to differentiate viral from bacterial causes. Hoarseness, cough, coryza, conjunctivitis, and diarrhea are classic features of a viral infection (Gereige and Cunill-De Sautu, 2011). Clinical Findings History The following may be reported: • Pain • Myalgia and arthralgia • Fever • Sore throat and dysphagia • Rhinitis, cough, hoarseness, stomatitis, stridor and conjunctivitis, nonspecific rash, or diarrhea points to a viral cause (Gereige and Cunill-De Sautu, 2011) • Acute onset of sore throat with headache, nausea, vomiting, and abdominal pain in the winter and early spring points to GABHS (Gereige and Cunill-De Sautu, 2011) Physical ExaminationCommon findings include: • Erythema of the tonsils and the pharynx • Reactive cervical lymphadenopathy Virus-specific physical findings include the following: • EBV can produce exudate on the tonsils, soft palate petechiae, and diffuse adenopathy. • Adenovirus can cause a follicular pattern on the pharynx (Cherry, 2009c). • Enterovirus can produce vesicles or ulcers on the tonsillar pillars and posterior fauces; coryza, vomiting, or diarrhea may be present. • Herpesvirus produces ulcers anteriorly and marked adenopathy. • Parainfluenza and RSVs cause more lower respiratory tract disease (e.g., croup, pneumonia, and bronchiolitis) with their typical respiratory signs of stridor, rales, or wheezing. • Influenza usually is associated with a cough, fever, and multiple systemic complaints. Diagnostic Studies A RADT and/or culture should be performed in children older than 3 years old with pharyngitis, because it is difficult to distinguish viral and streptococcal infections by history and physical examination. The incidence of GABHS is rare in children younger than 3 years; however, if there is a child in the household with a positive test for GABHS, children younger than 3 years can be tested (Shulman et al, 2012). In children and adolescents, negative RADT should be backed up by a throat culture, but positive tests do not need a backup due to high specificity of RADT testing (Gereige and Cunill-De Sautu, 2011; Shulman et al, 2012). This careful screening avoids the use of unnecessary antibiotics. Most viral causes of pharyngitis are benign, and other diagnostic studies are not usually used. Management For viral infection, only supportive care is needed, including fever and sore throat pain relief with acetaminophen or ibuprofen. Adequate fluid intake should be encouraged. Acute Bacterial Pharyngitis and Tonsillitis The most common bacterial cause of pharyngitis and tonsillitis in children and adolescents is GABHS, which accounts for about 15% to 30% of infections in children with acute sore throat and fever. Group C and group G streptococci can cause pharyngitis, but antibiotic treatment does not prevent its only nonsuppurative complication, glomerulonephritis (Shulman et al, 2012). A. haemolyticum is more common in adolescents but is difficult to culture, because the organism grows slowly (Martin, 2010). N. gonorrhoeae is a cause of adolescent pharyngitis if the patient engages in oral sex. M. pneumoniae and Chlamydophila pneumoniae are associated with cough along with pharyngitis. C. diphtheriae is an extremely rare cause of pharyngitis in the United States. See the GABHS discussion in Chapter 24. Clinical Findings History The following characterize GABHS infection: • Most commonly found in 5- to 13-year-old children; infrequent in children younger than 3 years old • Abrupt onset without nasal symptoms • Constitutional symptoms, such as arthralgia, myalgia, headache • Moderate to high fever, malaise, prominent sore throat, dysphagia • Nausea, abdominal discomfort, vomiting, headache • Presentation in late winter or early spring • Lack of a cough or nasal symptoms, along with an exudative, erythematous pharyngitis with a follicular pattern and typical historical findings point to GABHS Physical Examination The following may be seen in GABHS: • Petechiae on soft palate and pharynx, swollen beefy-red uvula, red enlarged tonsillopharyngeal tissue • Tonsillopharyngeal exudate that is yellow, blood-tinged (frequently) • Tender and enlarged anterior cervical lymph nodes • Bad breath • Stigmata of scarlet fever may be seen—scarlatiniform rash, strawberry tongue, circumoral pallor • Other bacterial infections may present with similar clinical findings: • A. haemolyticum causes an exudative pharyngitis with marked erythema and a pruritic, fine,scarlatiniform rash (Martin, 2010) • Adolescents and young adults with pharyngitis caused by A. haemolyticum may present with a scarlatiniform rash similar to scarlet fever • Variable presentation; may have mild pharyngeal erythema without tonsillar exudate or cervical adenopathy Diagnostic Studies It is important to use RADT for patients with clinical features consistent with GABHS to increase the yield of positive tests and avoid false-positive tests on patients who are carriers of streptococcus and do not need treatment. A RADT has a high specificity but variable sensitivity; therefore, a positive test indicates that a symptomatic person has strep infection and should be treated. However, a negative test does not mean that streptococcal infection is not present (Shulman et al, 2012). It is important not to do a strep test unless the patient has signs and symptoms, because a positive rapid strep test or a positive throat culture can identify a carrier state. Anti-streptococcal antibody titers, such as anti-streptolysin O (ASO) and anti-deoxyribonuclease B tests (anti-DNase B), are not useful in the diagnosis of acute pharyngitis, because the titers remain elevated for months after an acute infection. Anti-streptolysin O (ASO) is the most common test used to document past GABHS infection. ASO and anti-DNase B testing involves documenting antibody titers in response to streptolysin O or deoxyribonuclease B, respectively (Shulman et al, 2012). These two tests are often done together. The ASO titer rises 1 week postinfection and peaks 3 to 6 weeks after infection. Measurement of anti-streptococcal antibody titers is useful in the diagnosis of the nonsuppurative complications of GABHS, such as rheumatic fever or acute glomerulonephritis. The anti-DNase B test rises 1 to 2 weeks after infection, peaks 6 to 8 weeks following infection, and remains elevated for months even in the face of a mild infection with GABHS. As a result, these tests should not be used to diagnosis acute GABHS infection in a patient. Rare bacterial causes of pharyngitis that need treatment with antibiotics include C. diphtheriae and N. gonorrhoeae. Adolescents who have oral to genital sex with individuals who have N. gonorrhoeae of the genital region can develop pharyngitis from N. gonorrhoeae. A. haemolyticum is found in adolescents and young adults with a sore throat, and they may have a scarlatiniform rash similar to scarlet fever. These specimens need to be sent out for a culture with specific instructions to evaluate for these organisms. If infectious mononucleosis is suspected in a child, a complete blood count (CBC) can identify a lymphocytosis with atypical lymphocytes. This is a nonspecific test, because reactive (atypical) lymphocytes can occur in EBV, acquired CMV, viral hepatitis, rubella, roseola, and mumps (Jensen, 2011). Heterophile antibody testing can be helpful in school-age children and adolescents after the first week of infection but may yield a false negative in preschool and younger children (Gereige and Cunill-De Sautu, 2011). EBVspecific antibody testing is used to confirm acute or past infection. The presence of EBV immunoglobulin M (IgM), EBV IgG, and the late response (3 to 4 months) of antibodies to Epstein-Barr nuclear antigens (antiEBNA) helps to confirm the diagnosis of EBV. The presence of IgM antibody to the viral capsid antigen is the specific serologic test for EBV infection and generally confirms the diagnosis of acute infection; however, its presence is time sensitive (Jensen, 2011). Management Antibiotics should only be used in a symptomatic child with GABHS when the RADT or throat culture is positive due to increasing antibiotic resistance associated with overuse (Shulman et al, 2012). The goal of antibiotic therapy is to shorten the course and severity of illness, prevent the spread of illness to others, and prevent the development of suppurative and nonsuppurative complications. The use of beta-lactam antibiotics during an acute CMV or EBV infection causes a diffuse, morbilliform skin eruption (Schleiss, 2012) and should not be prescribed. It is important to treat these children within 9 days to prevent the nonsuppurative complication of rheumatic fever (Shulman et al, 2012). The management plan includes the following: • Antimicrobial therapy is based on positive tests results in a symptomatic patient (Shulman et al, 2012): • Penicillins (drugs of choice due to cost, efficacy, and infrequent adverse reactions): • Penicillin V potassium: Children (27 kg): 250 mg twice daily or three times daily; children (greater than 27 kg), adolescents, and adults: 500 mg twice daily or three times daily for 10 days; can do 250 mg four times a day with teens (Taketomo et al, 2014) • Amoxicillin suspension is more palatable (efficacy seems equal to penicillin): 50 mg/kg once daily (maximum dose = 1000 mg); alternate: 25 mg/kg (maximum dose = 500 mg) twice daily for 10 days• Benzathine penicillin G intramuscular (IM): 600,000 units as a single dose if less than 27 kg; 1.2 million units as a single dose for larger children and adults • If allergic to penicillin: • Pen Cephalexin: 20 mg/kg/dose twice daily (maximum dose = 500 mg/dose) for 10 days but should be avoided in patients with moderate hypersensitivity reaction to penicillin. • Cefadroxil: 30 mg/kg/day divided twice daily (maximum daily dose = 2 gms/day) for 10 days but should be avoided with moderate hypersensitivity reaction to penicillin. • Clindamycin: 7 mg/kg/dose three times daily (maximum dose = 300 mg/dose) for 10 days. • Azithromycin: 12 mg/kg once a day to a maximum of 500 mg. It should be noted that macrolide resistance is variable in the United States (Shulman et al, 2012). • Clarithromycin: 7.5 mg/kg/dose to a maximum of 250 mg twice per day for 10 days. • Supportive care: Antipyretics, fluids, and rest. • Use of corticosteroids is not indicated (Shulman et al, 2012). • Repeat culture is not generally needed except in situations where it is necessary to ensure eradication of the organism. • Continued symptoms of streptococcal pharyngitis and a positive culture for streptococcus may represent an actual treatment failure or a new infection with a different serologic type of streptococcus. • Noncompliance with pharmacologic therapy can explain treatment failure, and in these instances, an injection of benzathine penicillin is recommended. • Fomites, such as bathroom cups, toothbrushes, or orthodontic devices, may harbor GABHS and should be cleaned or discarded. • Children can return to school when they are afebrile and have been taking antibiotics for at least 24 hours. • Streptococcal treatment guidelines may include tonsillectomy as an intervention for recurrent GABHS; note that tonsillectomy should only be considered for the rare child who fails to improve over time despite good compliance (Shulman et al, 2012). This recommendation is different from the guidelines issued for tonsillectomy discussed earlier (Belderbos et al, 2011). • Treatment of chronic symptomatic carriage of GABHS: • Clindamycin: 20 to 30 mg/kg/day in three doses (maximum dose = 300 mg/dose) for 10 days • Amoxicillin-clavulanic acid: 40 mg/kg/day in three doses (maximum daily dose = 2000 mg/day) for 10 days • Penicillin V: 50 mg/kg/day in four doses for 10 days (maximum dose = 2000 mg/day) with the use of rifampin: 20 mg/kg/day in one dose (maximum dose = 600 mg/day) during the last 4 days of treatment • Benzathine penicillin G: 600,000 units for less than 27 kg and 1,200,000 units for 27 kg or greater; rifampin: 20 mg/kg/day in two doses (maximum dose = 600 mg/day) • If clinical relapse occurs, a second course of antibiotic is indicated, as discussed earlier. If recurrent infection is a problem, culturing of the family for the chronic carrier state is advised. Complications Major nonsuppurative late complications caused by GABHS are rheumatic fever, poststreptococcal reactive arthritis, and acute glomerulonephritis. Suppurative complications include cervical adenitis, rhinosinusitis, otitis media, pneumonia, mastoiditis, and retropharyngeal or peritonsillar abscess. Retropharyngeal abscess is more common in children younger than 6 years old, whereas peritonsillar abscess is more common from the ages of 20 to 40 years old (Gereige and Cunill-De Saut, 2011). Recurrent GABHS tonsillopharyngitis can also be a problem. Sydenham chorea is linked to GABHS infection. Pediatric autoimmune neuropsychiatric disorder syndrome (PANDAS) is characterized by various movement disorders, including tics, hyperactivity, paroxysmal and stereotypic motor movement, and psychiatric symptoms (e.g., obsessions and compulsions) and is associated with prior GABHS infection. There are five clinical criteria to diagnosis PANDAS. The child must have an (1) obsessive-compulsive disorder and/or other tic disorders; (2) onset of the disease must be prepubertal (3 years old to pubertal onset); (3) abrupt onset with a symptom course that is relapsing and remitting; (4) clear association with GABHS infection; and (5) neurologic abnormalities, such as motor hyperactivity or adventitious movements associated with exacerbations (Esposito et al, 2014). Due to the difficulties in establishing the temporal relationship between GABHS infection and the onset of neuropsychiatric symptoms and the lack of improvement with antibiotic therapy, it has been suggested that the diagnosis be changed to pediatric acute neuropsychiatric symptoms (PA6NS). This would require acomplete history and examination confirming the sudden acute onset of neuropsychiatric symptoms with therapies based on treatment of the symptoms (Swedo et al, 2012). Diphtheria Diphtheria is a rare infection of the respiratory tract caused by toxigenic strains of gram-positive C. diphtheriae or, less commonly, Corynebacterium ulcerans. The toxigenic strains produce two exotoxins— enzymatically active A domain and binding B domain. The binding B domain promotes entry of A into the cell. The bacterium has four biotypes (mitis, intermedius, belfanti, and gravis) that can be toxigenic or nontoxigenic. The ability of a strain of C. diphtheriae to produce toxin is related to bacteriophage infection of the bacterium, not to colony type. Humans are the only reservoir, and the organism is spread by respiratory droplets as well as contact with skin lesions. There have been no cases in the United States since 2003 (AAP, 2012). The bacteria can be shed for 2 to 6 weeks in an untreated patient. Disease may be mild or asymptomatic in partially or fully immunized individuals and severe if unimmunized. With toxin production, the primary infection can become lethal. Although rare, fomites can act as a vehicle of trans mission; and raw milk and milk products can transmit C. diphtheriae. Asymptomatic carriers can transmit the organism. The incubation period averages from 2 to 7 days but occasionally can be longer (AAP, 2012). The incidence of respiratory diphtheria is greater in the fall and the winter, but skin infections are more common in the summer. Endemic areas include Africa, Latin America, Asia, and the Middle East. In developing countries, a diphtheria-like illness caused by C. ulcerans is emerging (AAP, 2012). Clinical Findings History Infection is associated with a history of low-grade fever and gradual onset of symptoms over 1 to 2 days with bacterial shedding for 2 to 6 weeks if untreated. Fully immunized individuals can carry the bacteria asymptomatically and may present with a mild sore throat (AAP, 2012). Primary Infection • Low-grade fever • Grayish, adherent pseudomembrane found in either the nasopharynx, pharynx, or trachea • Bloody nasal discharge (with membranous pharyngitis is highly suggestive of diphtheria) (AAP, 2012) • Sore throat, serosanguineous or seropurulent nasal discharge, hoarseness, cough • Cutaneous lesions (nonhealing ulcers with dirty gray membrane or colonization of preexisting dermatoses) infected with diphtheria (seen less often) • Extensive neck swelling with cervical lymphadenitis, causing airway obstruction (due to membranous obstruction of the upper airway) • Possible otic and/or conjunctival infection findings Clinical indications of toxin production include the following: myocarditis and electrocardiographic changes, respiratory compromise, cranial nerve and local neuropathies, and peripheral neuritis. Diagnostic Studies A confirmatory diagnosis is based on a positive culture of C. diphtheriae. Specimens should be obtained from the nose, throat, any skin lesions, and either beneath the membrane or from a portion of the membrane. Because a special culture medium is needed, the lab needs to be notified if C. diphtheriae is suspected. Toxigenicity tests are performed if C. diphtheriae is confirmed. Culture results take 8 to 48 hours; however, treatment begins when diphtheria is suspected (AAP, 2012). Do not wait for laboratory confirmation. Results of the CBC may be normal or show a slight leukocytosis and thrombocytopenia. Differential Diagnosis Acute streptococcal pharyngitis and infectious mononucleosis are included in the differential diagnosis of pharyngeal diphtheria. A nasal FB or purulent rhinosinusitis can resemble nasal diphtheria; epiglottitis, laryngeal diphtheria, and viral croup can also cause obstruction. Management Children with diphtheria require hospitalization. Treatment consists of the following: • Antitoxin administration (hyperimmune equine antiserum): A single dose needs to be administered if there is a high index of suspicion prior to a positive culture result (AAP, 2015). Allergic reaction to the serum occursin 5% to 20%, so a scratch test should be performed prior to administration. IV immunoglobulin has not been approved for use. • Antimicrobial therapy: Erythromycin given orally or parenterally for 14 days, penicillin G for 14 days either IM or IV, or penicillin G procaine IM for 14 days. This is not a substitute for antitoxin administration. • Supportive care for respiratory, cardiac, and neurologic complications as appropriate. • Standard and droplet precautions until two cultures are negative. • Immunization after recovery because disease does not necessarily confer immunity. • Monitoring and antimicrobial prophylaxis of contacts regardless of immunization status. • Care for respiratory, cardiac, and neurologic complications. Complications and Prevention Peripheral neuropathy, myocarditis, acute tubular necrosis, and respiratory collapse can occur with severe disease. Universal immunization against diphtheria with regular booster injections is the only effective method of control. Infection can occur in immunized or partially immunized children, but the disease severity is greatly diminished in these individuals. Disease generally occurs in nonimmunized children; the frequency of severe life-threatening complications in this group is high. Care of a child exposed to diphtheria is individualized and based on immunization status, likelihood of follow-up, and compliance with antimicrobial therapy. The AAP Committee on Infectious Diseases' Red Book (2012) lists specific guidelines that should be followed for the care of exposed children. Pertussis Classic pertussis is either a primary disease or reinfection. Pertussis is caused by a gram-negative bacillus, Bordetella pertussis, of which there are six species. The most common types are B. pertussis and B. parapertussis (B. parapertussis causes a mild pertussis-like illness). B. bronchiseptica infrequently causes respiratory infection, and B. holmesii causes bacteremia. The last three bacteria are not affected by the vaccine. This infection is also known as whooping cough because of the high-pitched inspiratory whoop following spasms of coughing. B. pertussis produces a variety of components that are highly antigenic as well as biologically active. These include pertussis toxin (PT), adenylate cyclase toxin, dermonecrotic toxin, fimbriae, filamentous hemagglutinin, pertactin, and autotransporters. Pertactin, filamentous hemagglutinin, and fimbriae allow for bacterial adhesion, whereas PT inactivates the signaling pathways of the immune system, leading to a delay in recruitment of neutrophils. PT also causes the lymphocytosis and leukocytosis seen in the disease, as well as an activation of pancreatic beta cells leading to hypoglycemia. The B. pertussis irritates and inflames the ciliated epithelium lining, leading to tissue necrosis with epithelial cell damage from macrophages and reactive lymphoid hyperplasia (Snyder and Fisher, 2012). Transmission of these gram-negative pleomorphic bacilli is by aerosol droplet from coughing or from close contact with infected individuals. Contaminated droplets are inhaled and adhere to the ciliated epithelium of the nasopharynx. The incubation period is usually 7 to 10 days but can range from 5 to 21 days (AAP, 2015). Children are most contagious during the catarrhal stage and the first 2 weeks after the cough onset (AAP, 2012). The usual source of B. pertussis infection in infants is an unrecognized infection in an adult family member with a cough. The highest incidence of mortality occurs in infants less than 1 month old (Cherry and Heininger, 2009). The classic cough of pertussis lasts 6 to 10 weeks, but in 50% of adolescents it can last longer than 10 weeks. In infants under 6 months, there may be a short catarrhal stage and no whoop but a prolonged recovery period (AAP, 2012). Table 32-3 shows the stages of pertussis with accompanying symptoms. The cough is an attempt to dislodge plugs of necrotic bronchial epithelial tissue and thick mucus. Outbreaks of pertussis are increasing despite vaccination of adolescents and adults (Chiappini et al, 2013). Older children and adults are the likely vector of pertussis, because their disease may be mild or atypical in course (AAP, 2015). Up to 80% of household contacts acquire the disease from an index case due to waning immunization immunity and unrecognized disease. See Chapter 24 for information about the vaccine. Clinical Findings Manifestations of this disease vary by age group, stage of disease, immunization status, and the presence of transplacentally acquired antibodies (Cherry and Heininger, 2009). There is the classic illness and an asymptomatic infection. The latter occurs in patients who are vaccinated or as a primary illness in those who are not vaccinated. Characteristics of the classic disease in infants and young children are found in Table 32-3. Specific findings in infants younger than 6 months old are generally severe, particularly in neonates, and include: • Apnea (common) often with seizures caused by hypoxemia • Cough without an inspiratory whoop • Tachypnea • Poor feeding • Leukocytosis with a marked lymphocytosis (in the presence of persistent cough illness points to B. pertussis) Findings in children and adolescent may resemble the clinical course illustrated in Table 32-3 (Snyder and Fisher, 2012). Diagnostic Studies Although culturing for B. pertussis is considered the gold standard for pertussis (it is 100% specific), it should be noted that it is 12% to 60% sensitive, leading to missed diagnosis. It is difficult to do, because it requires collection from the nasopharynx with a Dacron or calcium alginate fiber-tipped swab placed immediately into a special transport medium. It is best done in the first 2 weeks post cough onset. Culture can be negative if the person has been ill for 2 weeks or more, has previously been vaccinated, or if antibiotics have been started (AAP, 2015). The organism is found most frequently during the catarrhal or early paroxysmal stage. Polymerase chain reaction (PCR) is increasingly popular due to its improved sensitivity (70% to 99%) and specificity (86% to 100%), as well as rapid result. PCR testing is done using a Dacron swab or nasal wash from the nasopharynx. However, the U.S. Food and Drug Administration (FDA) has not licensed any PCR tests or commercial kit for diagnostic use (AAP, 2012). The commercial tests using IgG to PT have not been FDA established, and IgM antibodies lack sensitivity and specificity (AAP, 2012). An increasing titer or a single IgG anti-PT has been used for diagnosis (AAP, 2012). As previously mentioned, leukocytosis and lymphocytosis are common findings in infants and young children, but they are rare in adolescents (AAP, 2012). Differential Diagnosis In the classic form of the disease, the diagnosis is clear due to the paroxysmal cough (Mueller et al, 2013). RSV can present with apnea. In infants, C. trachomatis is a differential diagnosis but tends to present with an interstitial pattern on chest x-ray (Snyder and Fisher, 2012). B. parapertussis, C. pneumonia, and M. pneumonia can cause a prolonged course in older children and adolescents. Adenoviruses, bocaviruses, and other viral agents can present with a cough illness. Gastroesophageal reflux, CF, asthma, and FBs should be included in the differential diagnosis. Management Treatment consists of the following: • Treatment with antimicrobial agents in the macrolide class is the treatment of choice. However, because the development of pyloric stenosis is linked with the administration of erythromycin in infants younger than 1 month, its use is discouraged in this age group (AAP, 2015). • Antibiotic treatment choices include (AAP, 2012; Taketomo et al, 2014): • Azithromycin: 10 mg/kg in a single dose for 5 days is given to infants from 1 to 6 months of age. • Azithromycin: 5-day treatment for infants older than 6 months of age, children, and adolescents, a single dose of 10 mg/kg/day (maximum dose of 500 mg) on day 1, then a single dose of 5 mg/kg/day (maximum dose of 250 mg) on days 2 to 5. • Clarithromycin: For infants, children, and adolescents, 15 mg/kg/day in two divided doses for 7 days with a maximum dose of 1 g/day. • Erythromycin: Used in all age groups except for infants younger than 1 month old; for infants 1 to 5 months: 10 mg/kg/dose, four times per day for 14 days; infants 6 months or older and children: 10 mg/kg/dose, four times per day for 7 to 14 days, with a maximum dose of 2 g/day; and adolescents: 500 mg four times a day for 7 to 14 days. • Trimethoprim (TMP)-sulfamethoxazole (SMX): Can be used as an alternative in infants older than 2 months who cannot tolerate macrolides at a dose of TMP 8 mg/kg/day and SMX 40 mg/kg/day in divided doses every 12 hours (maximum single dose 160 mg TMP). • Antimicrobial therapy given in the paroxysmal stage does not alter the course of pertussis, but does limit the spreading of the organism (AAP, 2012).• Corticosteroids should not be used. • Use of albuterol and other beta 2-adrenergic medications is not supported by controlled, prospective studies (Bocka, 2014). Care of Exposed Children Chemoprophylaxis is recommended for household and close contacts irrespective of immunization status. Early chemoprophylaxis in a household contact can limit secondary transmission. The value of chemoprophylaxis is limited after 21 days has elapsed but should be considered in high-risk household contacts (young infant, pregnant woman, or person who is in contact with infants) (AAP, 2012): • Immunization coverage with diphtheria-tetanus-acellular pertussis (DTaP) or Tdap depending on age group needs to be reviewed and updated. • Students and staff in schools need to be monitored for any respiratory symptoms with exclusion and evaluation for anyone with a cough illness. • Close monitoring of respiratory symptoms for 21 days after last contact with an infected individual. Complications The complication rate is higher in infants younger than 6 months old with a 1% mortality reported (Snyder and Fisher, 2012). Complications of pertussis include apnea in young infants, secondary bacterial pneumonia, seizures, encephalopathy, epistaxis, subconjunctival hemorrhage, syncope, sleep disturbances, rib fracture, incontinence, and death (AAP, 2012; Snyder and Fisher, 2012). Activation of tuberculosis is associated with pertussis infection. Prevention The concept of ―cocooning‖ around the infant is designed to protect the infants from dangerous or unwanted environmental hazards (Grizas et al, 2012; Healy et al, 2011). The recommendations of this strategy include targeting adult immunization by immunizing adults around the infant, including caretakers, grandparents over 65 years old, fathers, and mothers who were not immunized in the last trimester. In addition, all adolescents 11 to 18 years old should receive the Tdap vaccine. The infrastructure within maternity units as to immunization coverage has to be changed if ―cocooning‖ can take place in hospitals (Grizas et al, 2012). In addition, the recommended guidelines for the initial series of DTaP vaccines and booster doses should be followed. Remember that just one DTaP immunization can reduce the severity of symptoms in an infant infected with pertussis. See Chapter 24 for a discussion regarding this vaccine. The clinician needs to be mindful of valid contraindications to receiving pertussis vaccine. Immunity following either natural pertussis infection or illness or vaccination is not long lasting. Immunity wanes over time, and the vaccine does not confer active immunity. Pertussis in older children, adolescents, and adults is a mild, often unrecognized disease that if transmitted to an unimmunized infant can result in life-threatening illness. Universal immunization of children younger than 7 years old and adolescents is crucial to control this disease Restrictive Processes Restrictive disease is less common in pediatric patients and is characterized by decreased lung compliance with relatively normal flow rates. Examples of causative factors include neuromuscular weakness, lobar pneumonia, pleural effusion or masses, severe pectus excavatum, or abdominal distention. Key findings of restrictive lung disease are rapid respiratory rate and decreased tidal volume/capacity (Carter and Marshall, 2011). Defense Systems The respiratory defense system includes mechanical and biologic processes. Mechanical defenses include: • Filtering of particles • Warming and humidifying of inspired air • Clearing of airway through mucociliary and coughing actions • Spasm and breathing changes Approximately 75% of inspired air is warmed as it passes through the nose, paranasal sinuses, pharynx, larynx, and upper portion of the trachea. Final warming and humidifying of the airstream take place in the trachea and large bronchi. Heat and moisture are removed during the expiratory phase of respiration. The nose has a large surface area on which particles larger than 5 mm are trapped and filtered to prevent them from entering the lower airways. The trachea and bronchioles are lined with various defensive cells and mucus glands. Goblet cells secrete the mucous layer that lies on the tip of cilia. Particles entering the conducting airway are quickly cleared by the mucociliary defenses.Coughing is a reflex mechanism that has three phases: (1) inspiratory, (2) compressive, and (3) expiratory. Through forceful expiration FBs and other materials can be removed from the airways; coughing propels particles. Young infants and children cannot effectively expectorate mucus, so they swallow it. Loss of the cough reflex leads to aspiration and pneumonia. Temporary breathing cessation, reflex shallow breathing, laryngospasm, and even bronchospasm are compensatory efforts aimed at stopping foreign matter from further entry into the lower respiratory tract. 797However, these respiratory efforts offer limited protection and have significant drawbacks. Biologic processes that protect the respiratory system include: • Phagocytosis • Absorption of noxious gases in the vasculature of the upper airway • Absorption of particles by the lymph system Phagocytosis, aided by the secretory IgA plus interferon, lysozyme, and lactoferrin, is the principal antimicrobial defense. Particles reaching the alveoli can be phagocytized by alveolar macrophages and polymorphonuclear (PMN) cells, cleared from the lung by the mucociliary system, or carried by lymphocytes into regional nodes or the blood. These particles can take days to months to clear. The respiratory defense system is at risk for compromise from numerous environmental factors. Damage to epithelial cells is caused by a variety of substances and gases, such as sulfur, nitrogen dioxide, ozone, chlorine, ammonia, and cigarette smoke. Hypothermia, hyperthermia, morphine, codeine, and hypothyroidism can adversely alter mucociliary defenses. Dry air from mouth breathing during periods of nasal obstruction, tracheostomy placement, or inadequately humidified oxygen therapy results in dryness of the mucous membrane and slowing of the cilia beat. Cold air is also irritating to the lower airways. Phagocytic ability is also reduced by many substances, including ethanol ingestion and cigarette smoke. Hypoxemia, starvation, chilling, corticosteroids, increased oxygen, narcotics, and some anesthetic gases also impair phagocytosis. Recent acute viral infections can reduce antibacterial killing capacity. Damage from infection and chemical irritants may or may not be reversible. Recurrent respiratory infections in children merit investigation for immunodeficiency or other underlying diseases, such as primary ciliary dyskinesia or CF. The mnemonic SPUR (Bush, 2009) can help determine which children need further workup: Severe infection Persistent infection and poor recovery Unusual organisms Recurrent infection Immunodeficiencies should be considered if the child has four or more new ear infections in a year, two or more serious sinus infections, two or more pneumonias in a year, persistent oral candidiasis, failure to thrive, two or more deep seeded skin abscesses, 2 or more months on antibiotics without improvement, and/or the need for intravenous (IV) antibiotics to clear infections. Also consider immunodeficiencies if there is a family history of immunodeficiency or two or more deep skin infections (Modell et al, 2014). Assessment of the Respiratory System The history provides valuable information about the causes, progression, and potential complications of a child's respiratory condition. The physical examination and diagnostic testing allow the provider to determine the extent of respiratory distress. History History of the present illness can be assessed using the mnemonic PQRST: • Promoting, preventing, precipitating, palliating factors • Contacts: Are any family members or close contacts (e.g., day care, school) ill with similar signs and symptoms? • Prevention: Do you give your child any medications or supplements (include any herbs, botanicals, or vitamins) to try to prevent a cold? What are your hand washing practices? Do you encourage fluids when your child has a URI? Are the child's immunizations up to date? • Progression: Are the respiratory signs or symptoms increasing in severity, lessening, or about the same? Is the child easily fatigued, less active, having trouble sleeping, or working harder to breathe? • Treatment: Have any OTC, prescription drugs, herbs, supplements, or botanicals been used? Have any other treatment modalities been used, including folk cures or home remedies? • Quality or quantity • How severe are the symptoms? Is the illness interfering with school attendance or play? Are breathing problems affecting the child's ability to sleep and eat? • Region or radiation • Does the child complain of chest pain? • Severity, setting, simultaneous symptoms or similar illnesses in the past • Key signs and symptoms: Has the child had symptoms or signs of a daytime or nighttime cough, fever, vomiting, malaise, rhinorrhea, sore throat, lesions in the mouth, retractions, cyanosis, dyspnea, or increased respiratory effort? Table 32-1 lists key characteristics and causes of cough. TABLE 32-1 Key Characteristics of Cough, Common Causes, and Questions to Ask in a Pediatric HistoryKey Characteristics to Consider Description and Questions to Ask Age factor Infants have a weak, nonproductive cough. Quality Staccato-like (Chlamydia trachomatis in infants); barking or brassy (croup, tracheomalacia, habit cough); paroxysmal or inspiratory whoop (pertussis or parapertussis); honking (psychogenic). Is the cough wet or dry? Duration Acute (most causes are infectious and last less than 2 weeks), subacute (cough lasts from 2 to 4 weeks); recurrent (associated with allergies and asthma), or chronic (lasting greater than 4 to 8 weeks [e.g., CF, asthma]). Is the cough continuous or intermittent? Productivity Mucus producing or nonproductive? Timing During the day, night (associated with asthma), or both? Effect on parent and child Are parents frustrated with the cough? Is it causing them to lose sleep and work time? Are they concerned that the child may have something serious? Associated symptoms Fever: May indicate bacterial infection (pneumonia). Rhinorrhea, sneezing, wheezing, atopic dermatitis: Associated with asthma and allergic rhinitis. Malaise, sneezing, watery nasal discharge, mild sore throat, no or low fever, not ill appearing: Typical of URI. Tachypnea: Pneumonia or bronchiolitis in infants (infants may not have a cough). Exposure to infection or travel Has the child been out of the country (tuberculosis)? Is there a member of the household being treated for “bronchitis” or another cough illness?Key Characteristics to Consider Description and Questions to Ask Causes Congenital anomalies Tracheoesophageal fistula, vascular ring, laryngeal cleft, vocal cord paralysis, pulmonary malformations, tracheobronchomalacia, congenital heart disease Infectious agent Viral (RSV, adenovirus, parainfluenza, HIV, metapneumovirus, human bocavirus), bacterial (tuberculosis, pertussis, Streptococcus pneumoniae), fungal, and atypical bacteria (C. and M. pneumoniae) Allergic condition Allergic rhinitis, asthma Other FB aspiration, gastroesophageal reflux, psychogenic cough, environmental triggers (air pollution, tobacco smoke, wood smoke, glue sniffing, volatile chemicals), CF, drug induced, tumor, congestive heart failure CF, Cystic fibrosis; FB, foreign body; HIV, human immunodeficiency virus; RSV, respiratory syncytial virus; URI, upper respiratory infection. Adapted from Chang AB: Cough, Pediatr Clin North Am 56(1):19–31, 2009; Cherry JD: Croup (laryngitis, laryngotracheitis, spasmodic croup, laryngotracheobronchitis, bacterial tracheitis, and laryngotracheobronchopneumonitis). In Cherry J, Kaplan S, Demmler-Harrison G, et al, editors: Feigin & Cherry's textbook of pediatric infectious diseases, ed 6, vol 1, Philadelphia, 2009, Saunders/Elsevier, pp 254–268. • Associated symptoms: Has there been a decrease in appetite or feeding? Any rashes, headaches, or abdominal pain? • Similar illnesses in the past: Does the child have a history of respiratory tract infections, allergies, or asthma? How many similar infections has the child had (e.g., croup, pneumonia, rhinosinusitis, streptococcal tonsillopharyngitis, or frequent colds)? • Temporal factors • When did the illness begin? • Was the onset acute or insidious or proceeded by the common cold? • How long has it lasted? How has it changed over time? • Family history • Do others in the family have a history of allergies or asthma? 798 • Is there any family history of immunodeficiency, ear-nose-throat, or respiratory problems? • Does anyone in the family have genetic diseases, such as CF or alpha 1-antitrypsin deficiency? • Are other family members ill? • Review of systems • Note any infections, constitutional diseases, or congenital problems that might have a respiratory component. • Environment • Does anyone in the family or in the day care setting smoke? Does the child live or attend school in an urban or industrial area subject to air pollution (e.g., near a major highway, industrial plant, or bus terminal)? Has the child or a family contact traveled recently and where? Physical Examination When determining respiratory distress, think about the total presentation and not just individual isolated findings. Consider the anxiety level, respiratory rate and rhythm, use of accessory muscles, color, breath sounds, grunting, and pulse oximetry results. Information pertinent to the physical examination of a child with suspected respiratory disease includes the following: • Measurement of vital signs and observation of general appearance: • A normal respiratory rate is age dependent and, if elevated, is a key indicator of lower respiratory involvement.• The level of anxiety, nasal flaring, and position of comfort are useful indicators of respiratory distress. 799Changes in skin color may be subtle or obvious, depending on the level of deoxygenation. Grunting is a sign of small airway disease. • Inspection of: • Nose: Look for rhinorrhea—clear, mucoid, mucopurulent; FBs, erosion, polyps, lesions, bleeding, septal position, and color of the mucous membrane. • Throat, pharynx, and tonsils: Look for lesions, vesicles, exudate, enlargement of any structure, or other abnormalities. If epiglottitis is a consideration, do not inspect the mouth or attempt to elicit a gag reflex (see discussion later in this chapter). • Chest: Look at the depth, ease, symmetry, and rhythm of respiration. These are key indicators of lower respiratory tract involvement. The use of accessory muscles and the presence of retractions should be noted. A prolonged expiratory phase is associated with respiratory obstruction in the lower airways. • Palpation or percussion of: • Chest: Percuss for signs of dullness or hyperresonance caused by consolidation, fluid, or air trapping. • Auscultation of the chest: • Upper tract: Pathology frequently causes noisy breathing, snoring, stridor, and musical or wheezing tracheal breath sounds and can be a source of referred breath sounds (Bohadana et al, 2014). • Lower tract: Pathology is suggested by fine crackles, coarse crackles, rhonchus, pleural friction rub, wheezing, and bronchial breath sounds (Bohadana et al, 2014). Diagnostic Studies Diagnostic procedures used to evaluate respiratory illness in children managed as outpatients include the following: • Monitoring oxygenation by pulse oximetry: • Pulse oximetry can be used to continuously measure pulse rate and peripheral oxygen saturation in arterial blood. The oxyhemoglobin saturation percentage (SpO2) is digitally displayed. Results generally correlate well with simultaneous arterial oxygen saturation (SaO2). With anoxia, there is a rise in organic phosphate content within the red blood cells (RBCs) resulting in more oxygen (O2) available to tissues. People living at higher elevations suffer from chronic hypoxia. When first arriving at a high elevation, many individuals experience a transient mountain sickness with symptoms that include headache, insomnia, irritability, breathlessness, nausea, and vomiting. This phenomenon lasts approximately 1 week before acclimatization begins. The affected person begins to increase production of RBCs. Finally, a functional nonpathologic right ventricular hypertrophy takes place. These effects last as long as the person remains at high elevation. Severe altitude sickness can lead to cerebral and pulmonary edema and can be life-threatening. • Blood gas studies can help the provider assess possible respiratory collapse and are used in acute care settings. A rising partial arterial pressure of carbon dioxide (PaCO2) is an ominous sign. • Radiographic imaging in respiratory disease may be efficacious in certain circumstances. Such imaging can include radiographs, ultrasonography, magnetic resonance imaging (MRI), and computed tomography (CT) of the sinuses, soft tissues of the neck, and chest. Abnormalities of the nasal mucosa, such as thickening, may reflect inflammation. Unless there is chronic or complicated rhinosinusitis, imaging in acute rhinosinusitis (ARS) is not indicated, because uncomplicated URIs can cause abnormalities of the paranasal sinuses (Brook, 2013). Chest radiographs should be done in both posteroanterior and lateral positions, because lesions may only be seen in one of the two views. Other pulmonary studies may be ordered by the medical specialists to whom the child is referred. Pulmonary function tests are discussed in Chapter 25 in the section on asthma. • Other specialized tests, including sweat testing, cultures, and blood work, are addressed under the specific illness. • Children who have unusual signs and symptoms should be referred to a pulmonary specialist at which time further diagnostic procedures might be performed. Fluoroscopy is useful in the evaluation of stridor and abnormal movement of the diaphragm. Endoscopy (bronchoscopy and laryngoscopy), bronchoalveolar lavage, percutaneous tap, lung biopsy, and microbiology studies can be helpful if used appropriately. Contrast studies (e.g., barium esophagogram) are useful for patients with recurrent pneumonia, persistent cough, tracheal ring, or suspected fistulas. Other imaging studies that might be needed to assess these children include bronchograms (useful in delineating the smaller airways), pulmonary arteriograms (evaluation of the pulmonary vasculature), and radionuclide studies (evaluation of the pulmonary capillary bed).Upper Respiratory Tract The upper respiratory tract includes the nostrils, nasopharynx, larynx, upper part of the trachea, eustachian tubes, and sinuses. Air is warmed and humidified as it travels through the nasal passages, and coarse nasal hairs filter out particles. The nasal passages are lined with lysozymes, secretory immunoglobulin A (IgA), and immunoglobulin G (IgG) in nasal mucosa to defend against microbial invasion. The nasal mucosa is continuous and similar to the sinus mucosa except that the nasal mucosa is thicker with more glands. A blanket of mucus covers the surface epithelium of the nasal and sinus mucosa. The mucosal lining of the sinuses is composed of pseudostratified, ciliated columnar epithelium interspersed with mucus producing goblet cells (Rose et al, 2013). The mucociliary action of the paranasal epithelium moves secretions from the sinuses to the nasal cavity. The frontal, maxillary, and anterior parts of the ethmoid sinuses drain to the middle meatus of the nose, whereas the sphenoid and posterior parts of the ethmoid sinuses drain to the superior meatus of the nose. Secretions need to be able to move through patent ostia into the nose. The quality of secretions and normally functioning cilia are key factors in the movement of secretions into the nose. Inflammation of nasal mucosa frequently causes edema and disruption of the sinus secretions. If there is significant swelling of the ostia due to a URI or allergic inflammation, or mechanical or local obstruction, ostial obstruction results and obstruction of the sinus secretions occurs. Cilia movement and mucus flow allow the sinuses to be free of pathogens. The maxillary sinuses are present by the second trimester of gestation but are not fully pneumatized until a child is about 4 years old. Ethmoid sinuses develop by the fourth month of gestation and form the thin lateral walls of the orbit of the eye. They are pneumatized at birth and can be visualized on plain radiographs when the child is 1 to 2 years old. The sphenoid sinuses start to form in the first 2 years of life but remain rudimentary until age 6, which is when they become visible on radiographs. By 12 years old, they reach their permanent size, but not shape. As a result, the nasal cavity and paranasal sinuses reach adult proportion by age 12 (Cherry and Shapiro, 2009). The sinuses become clinically significant sites of infection at the following ages: • Maxillary and ethmoid sinuses: As early as late infancy • Sphenoid sinuses: Around 3 and 4 years old • Frontal sinuses: Around 6 to 10 years old The epiglottis deflects swallowed material toward the esophagus to protect the larynx. The vocal cords form a V-shaped opening known as the glottis.The subglottic space is beneath the vocal cords, and its walls converge toward the cricoid ring to form a complete ring of cartilage around the larynx. In children younger than 2 to 3 years old, the cricoid ring is the narrowest part of the airway; in older children and adults, the glottis is narrowest. The rings of tracheal cartilage support the trachea and the mainstem bronchi. 795 The trachea and airways of the infant and young child are more compliant than those of an adult. Hyperextension of the neck can constrict the airway of infants. Consequently, changes in intrapleural pressure lead to greater changes in an infant's or young child's airway compared with the effect that such changes would exert on adult airways, thereby causing an increased risk of airway collapse. Similarly, increased chest wall compliance in young infants makes them more vulnerable to adverse events, and their respiratory muscles cannot effectively handle sustained, intense respiratory workload that occurs during severe pulmonary illnesses. Bronchiolitis Bronchiolitis is also called infectious asthma, asthmatic bronchitis, wheezy bronchitis, or virus-induced asthma. Bronchiolitis is a disease that causes inflammation, necrosis, and edema of the respiratory epithelial cells in the lining of small airways, as well as copious mucus production (Ralston et al, 2014). Bronchiolitis is characterized by the insidious onset of URI symptoms over 2 to 3 days that progresses to lower respiratory symptoms that last as long as 10 days (Da Dalt et al, 2013). It is a communicable disease found primarily in infancy to 2 years old (Teshome et al, 2013) that accounts for 10% of visits to a primary provider the first 2 years of life (Schroeder and Mansbach, 2014). Bronchiolitis is a common diagnosis used for an infant seen with wheezing for the very first time and is the leading cause of hospitalizations for infants. The most common age for severe disease occurs in infants between 2 to 3 months due to the natural postnatal nadir in maternal immunoglobulins received via the placenta during the last trimester (Da Dalt et al, 2013). More than 80% of the cases of bronchiolitis occur in infants younger than 1 year of age with a male-to-female ratio of 1.5 : 1 (Welliver, 2009). In mild cases, symptoms can last for 1 to 3 days. In severe cases, cyanosis, air hunger, retractions, and nasal flaring with symptoms of severe respiratory distress within a few hours may be seen. Apnea can occur with a wide range of prevalence reported (Ralston et al, 2014) and may require mechanical ventilation. Newer understanding of the pathophysiology in bronchiolitis points to airway obstruction as a result of epithelial and inflammatory cellular debris due to infiltration of the virus into the small bronchiole epithelium and alveolar epithelial cells (AEC), types I and II. Membranous pneumatoceles, or AEC type I, are dominant and cover 96% of the respiratory tree. Their role is in gas exchange, whereas AEC type II are important to surfactant production (Chuquimia et al, 2013). It is a disease of the small bronchioles that are 2 mm in size. There is a sparing of basal cells in the bronchiole. The main lesion is epithelial necrosis, which leads to a dense plugging of the bronchial lining. This results in increased airway resistance, atelectasis, hyperinflation, and increased mucus production (Teshome et al, 2013).Bronchiolitis is a viral illness predominantly caused by RSV, especially in outbreaks (Da Dalt et al, 2013; Welliver, 2009). Recent data suggest that up to 30% of infants with severe bronchiolitis are co-infected with two or more viruses (Mansbach et al, 2012). In descending order after RSV, rhinovirus, parainfluenza, adenovirus, and mycoplasma are causes (Teshome et al, 2013). Metapneumovirus was discovered in 2001 and is a cause of bronchiolitis 7% of the time. Human bocavirus is a common co-infecting virus with RSV and is found up to 80% of the time (Teshome et al, 2013). RSV-specific immunoglobulin E (IgE), eosinophils, and chemokines may play a role in the pathogenesis of bronchiolitis (Welliver, 2009). Adenovirus and RSV can cause long-term complications. The incubation period for RSV is 2 to 8 days and typically occurs from November through March with virtually no outbreaks in the summer (Teshome et al, 2013; Welliver, 2009). Fever tends to be higher with adenovirus versus RSV (Teshome et al, 2013). Respiratory viruses are spread by close contact with infected respiratory secretions or fomites and can live on 818surfaces for up to 30 minutes (Teshome et al, 2013). The most frequent mode of transmission is hand carriage of contaminated secretion. The source of infection is an older child or adult family member with a ―mild‖ URI. Older children and adults have larger airways and tolerate the swelling associated with this infection better than infants do. Most cases of bronchiolitis resolve completely, but recurrence of infection is common, and symptoms tend to be mild. Infants who are at higher risk of severe RSV include children with major chronic pulmonary disease, such as CF, neuromuscular disorders, or bronchopulmonary dysplasia; premature birth before 35 weeks of gestational age; and infants with significant hemodynamically difficulties due to congenital heart disease (Teshome et al, 2013). Other risk factors for severe RSV disease are male gender, crowded household, lack of breastfeeding, smoke exposure, day care attendance, having siblings, birth during the winter months, and immunodeficiency (Da Dalt et al, 2013). Clinical Findings History The following are reported: • Initial presentation: Typically the illness begins with URI symptoms of cough, coryza, and rhinorrhea and progresses over 3 to 7 days (Smith, 2011). • Gradual development of respiratory distress marked by noisy, raspy breathing with audible expiratory wheezing. • Low-grade to moderate fever up to 102° F (38.9° C). • Decrease in appetite. • No prodrome in some infants; rather they have apnea as the initial symptom. • Usually the patient's course is the worst by 48 to 72 hours after the wheezing starts and then the patient starts to improve. If the child has a bacterial illness, the child will continue to worsen with a high fever. Physical Examination Findings include the following: • Upper respiratory findings • Coryza • Mild conjunctivitis in 33% (Welliver, 2009) • Pharyngitis • Otitis media in up to 15% (Welliver, 2009) • Lower respiratory findings (Teshome et al, 2013) • Tachypnea (approximately 40 to 80 breaths per minute) • Substernal and/or intercostal retractions • Heterophonous expiratory wheezing • Fine or coarse crackles may be heard throughout the breathing cycle • Varying signs of respiratory distress and pulmonary involvement (e.g., nasal flaring, grunting, retractions, cyanosis, prolonged expiration) • Abdominal distention • Palpable liver and spleen, pushed down by hyperinflated lungs and a flattened diaphragm Diagnostic Studies A diagnosis of bronchiolitis should be based on the history and physical examination (Ralston et al, 2014). Overuse of diagnostic testing persists in clinical practice despite available guidelines on the diagnosis and management of bronchiolitis (Librizzi et al, 2014; Ralston et al, 2014; Turner et al, 2014). The routine use of chest radiographs in previously healthy infants with mild RSV bronchiolitis is not indicated. Evidence-based guidelines from the AAP and the Scottish Intercollegiate Guidelines Network (SIGN) are strongly against routine chest radiography, including those in previously healthy infants with mild RSV bronchiolitis (Ralston et al, 2014; SIGN, 2006). In severe illness, a chest x-ray may be ordered to rule out pneumonia or pneumothorax, but its use must be weighed against the dangers of radiation exposure. The findings of chest radiography can vary, and even with severe illness the x-ray can be clear with a flattened diaphragm and an increase in anteroposterior diameter. Areas of atelectasis can appear like a pneumonitis, but true pneumonia is uncommon (early bacterial pneumonia can be difficult to detect and cannot be ruled out by radiographs).Routine virologic testing is not recommended. In selected situations (hospitalization or if an infant has received monthly palivizumab [Synagis]), enzyme-linked immunosorbent assays or fluorescent antibody techniques to look for RSV are the diagnostic procedures of choice in most laboratories. Viral culture of nasal washings can be done in severe cases to confirm RSV, parainfluenza viruses, influenza viruses, and adenoviruses. PCR is helpful in deciding about isolation of cohorts with the same infection in the hospital setting. The cost of the diagnostic viral testing may outweigh the clinical usefulness of knowing which virus is infecting the patient. Hematologic testing is not recommended in the latest guidelines. If a CBC is done for another reason, a mild leukocytosis may be seen with 12,000 to 16,000/mm3. Routine laboratory tests are usually not required to confirm the diagnosis, because they lack specificity. However, young infants pose a diagnostic dilemma, because they are at greater risk of a serious bacterial infection (SBI) and, therefore, blood cultures and CBC with differential are done with a higher rate of antibiotic use in infants who had these blood tests (Librizzi et al, 2014). Urine cultures actually have a higher rate of positive results in the young febrile infant (up to 2.3% in a bronchiolitis study conducted by Librizzi and colleagues). Differential Diagnosis The diagnosis of bronchiolitis can be confused with asthma, but there are some differences that may be helpful. Asthma is an acute process due to airway hyperreactivity and inflammation, whereas the onset of bronchiolitis is insidious. The response to the usual asthma therapies of beta agonist and 819steroids is poor in infants with bronchiolitis. In contrast, certain viral illnesses in young children can induce wheezing that will respond to a β-agonist with good results. FB aspiration is discussed in greater detail later in this chapter, but this is usually a toddler with a history of choking who then develops focal areas of wheezing. Although children with congestive heart failure can wheeze, they also show symptoms of sweating and the signs of failure to thrive with a murmur and an S4 gallop rhythm. Other differentials include airway irritants, gastroesophageal reflux, pneumonia, allergic pneumonitis, vascular rings, lung cysts, and lobar emphysema (Teshome et al, 2013; Welliver, 2009). Management Evidence-based guidelines published by the AAP no longer support a trial of bronchodilators as an option for infants and children with bronchiolitis because of the risk associated with its use and the lack of evidence of an effect (Ralston et al, 2014; Schroeder and Mansbach, 2014). The use of epinephrine is also not recommended for infants and children. Administration of nebulized hypertonic saline to infants in the emergency department is not recommended; however, nebulized hypertonic saline can be administered to infants and children diagnosed with bronchiolitis and hospitalized. Systemic corticosteroids should not be administered in the treatment of bronchiolitis in infants; chest physiotherapy is contraindicated in infants and children. Antibiotics have no place in the treatment of a viral disease (such as, bronchiolitis), unless there is a concomitant bacterial infection or strong suspicion. Most infants with mild signs of respiratory distress can be treated as outpatients if their oxygen level is within a normal range (Ralston et al, 2014; Schroeder and Mansbach, 2014): • Supportive care consists of adequate hydration and use of antipyretics. • The need for supplemental oxygen administration is based on oxyhemoglobin saturation levels. If an infant's or child's oxyhemoglobin level is greater than 90%, the decision to administer oxygen is left up to the provider (Ralston et al, 2014). • Transcutaneous oxygen saturation monitoring (continuous pulse oximetry) is also an individual provider's choice (Ralston et al, 2014). • Fluid intake is strongly recommended to prevent dehydration. • Nasal suctioning to clear the upper nasal passages is recommended. The inpatient management of bronchiolitis may include using heated, humidified, high-flow oxygen via nasal canula. The mechanism of action is to improve mucous ciliary clearance and avoid nasal dryness. The high flow delivers positive airway pressure to keep the alveoli open and reduce ventilation perfusion mismatch and small airway microatelectasis (Da Dalt et al, 2013). This method needs more research but is being regularly used in the inpatient basis (Schroeder and Mansbach, 2014; Teshome et al, 2013). Hypertonic saline (3%) is being used to treat bronchiolitis in hospitalized infants and children. The mechanism of action is due to decreasing mucus viscosity, thus improving airway clearance. It is not recommended for outpatient use and does not reduce hospital admission in patients being treated in the emergency department. However, its use does reduce length of hospital stay (Da Dalt et al, 2013; Zhang et al, 2008). Research on this method is ongoing. The use of deep airway suctioning is avoided, though continuing to keep the nasal airway clear on a regular basis may improve airflow. This intervention is intuitive and does not need a randomized trial to show its benefit (Schroeder and Mansbach, 2014). As stated previously, there is no evidence for the routine use of antibiotics, β-agonist, or corticosteroids. Ribavirin is no longer recommended routinely and is presently only used in infants with severe illness due to underlying immunodeficiency, chronic lung disease, or hemodynamically unstable cardiac conditions (Da Dalt et al, 2013). Although leukotriene levels are high in bronchiolitis, the use of antileukotriene inhibitors has not been adequately studied and, thus, is not recommended. A recent review showed an increased risk of bronchiolitis with low cord blood vitamin D level (Belderbos et al, 2011). At present, there is no evidence to show any pharmacologic therapy is clearly superior. Parents caring for infants and children at home need to understand: • The management of rhinitis (use of saline drops and suctioning of nares) • Indications for the use of antipyretics • The use of home oxygen • Signs of increasing respiratory distress or dehydration that call for hospitalization • Guidelines for feeding an infant with signs of mild respiratory distress (amount of fluid needed per 24 hours; smaller, more frequent feedings; monitoring of the respiratory rate; and guarding against vomiting) • Education that infants and children with bronchiolitis typically have symptoms for 2 to 3 weeksInfants younger than 2 months old and older infants with signs of severe respiratory distress should be hospitalized. Signs that suggest increasing respiratory distress include the following (Smith, 2011): • Progressive stridor or stridor at rest • Apnea • Increasing respiratory rate (sleeping rate of greater than 50 to 60 breaths per minute) • Restlessness, pallor, or cyanosis • Hypoxia recorded by either blood gas (partial pressure of oxygen [PO2] less than 60 mm Hg) or pulse oximetry (less than 92% on room air) • Rising partial pressure of carbon dioxide (PCO2) (recorded by blood gas) • Inability to tolerate oral feedings • Depressed sensorium 820 • Presence of chronic cardiovascular or immunodeficiency disease • Parent unable to manage at home for any reason In-hospital management focuses on supportive care, focusing on suctioning of nares, humidified supplemental oxygen, and elevation of the child to a sitting position at a 30- to 40-degree angle. IV hydration (or in infants nasogastric hydration) is needed when respiratory distress interferes with nursing or bottle feeding. Occasionally a hospitalized child is not able to be quickly weaned back to room air. Home management of these infants requiring oxygen is sometimes difficult and may require a team approach, including involvement of a pediatric health care provider and home care nursing visits. Strict outpatient follow-up is mandatory for as long as the child is receiving home oxygen. Complications The first 48 to 72 hours after the onset of cough are the most critical. Apneic spells are common in infants. The child is ill-appearing and toxic but gradually improves. The fatality rate associated with bronchiolitis is about 1% to 2%. Infants younger than 12 weeks old and those with underlying cardiorespiratory or immunodeficiency are at risk for severe disease. Prolonged apnea, uncompensated respiratory acidosis, and profound dehydration secondary to loss of water from tachypnea and an inability to drink are the factors leading to death in young infants with bronchiolitis. In some children, bronchiolitis can cause minor pulmonary function problems and a tendency for bronchial hyperreactivity that lasts for years. RSV bronchiolitis has been associated with the development of asthma, but its role in the causality of asthma is still debated. Recurrent episodes of wheezing can be seen during childhood in patients with a history of bronchiolitis. This persists into adolescence with 10% of the children still wheezing. However, this figure may not be different from the general population (Welliver, 2009). Prevention Palivizumab (Synagis) is an RSV-specific monoclonal antibody used to provide some protection from severe RSV infection for high-risk infants (see Chapter 24 for guidelines). Educate caregivers about decreasing exposure to and transmission of RSV, especially those with high-risk infants. Advice should include limiting exposure to child care centers whenever possible; use of alcohol-based hand sanitizers if available or hand washing if the alcohol-based hand sanitizer is not available (Ralston et al, 2014); avoiding tobacco smoke exposure; and scheduling RSV prophylaxis vaccination, when indicated. Foreign Body Aspiration The symptoms and physical findings associated with aspiration of an FB depend on the nature of the material aspirated, plus the location and degree of the obstruction. The cough reflex protects the lower airways, and most aspirated material is immediately expelled with coughing. Onset of a sudden episode of coughing without a prodrome or signs of respiratory infection should make the provider suspicious of FB aspiration. Objects that are either too large to be eliminated by the mucociliary system or cannot be expelled by coughing eventually lead to some form of respiratory symptomatology. Obviously a large FB occluding the upper airway can cause suffocation. A small object in the lower respiratory tree may not produce symptoms for days to weeks. Obstruction results from either the FB itself or edema associated with its presence. Hot dogs are one of the most common causes of fatal aspiration. Although toddlers commonly aspirate FBs, aspiration occurs in children of all ages. Laryngeal Foreign Body Clinical Findings History. A rapid onset of hoarseness and the development of a chronic croupy cough with aphonia are reported. Be suspicious of an FB aspiration in children with sudden episode of cough, unilateral wheezing, and or recurrent pneumonia. Physical Examination. The child can present with cough, unilateral wheezing, clinical signs of pneumonia, hemoptysis, dyspnea, and cyanosis.Diagnostic Studies. Expiratory or lateral decubitus chest radiographs should be ordered. Because most FBs are not radiopaque, radiographs may not be useful in the diagnosis. However, if a chest radiograph does not reveal an FB but shows local emphysema—an area that does not inflate or deflate— suspect FB aspiration (Carter and Marshall, 2011). If the history suggests FB aspiration, bronchoscopy must be undertaken. Direct laryngoscopy might reveal the presence of foreign matter. Tracheal Foreign Body Clinically the child has a history of a brassy cough, hoarseness, dyspnea, and possibly cyanosis. The most characteristic signs of tracheal FB aspiration are the homophonic wheeze and the audible slap and palpable thud sound produced by the momentary expiratory effect of the FB at the subglottic level. Bronchial Foreign Body Most objects are aspirated into the right lung. A careful medical history may reveal a forgotten episode of choking. Clinical Findings History. An initial episode of coughing, gagging, and choking is described. Some objects are inhaled with no choking (e.g., a spear of grass). Bloodstreaked sputum may be expectorated, but hemoptysis rarely occurs as an early symptom. On rare occasions hemoptysis does occur as an initial symptom months or years after the aspiration event took place. Children aspirating a metallic object often complain of a ―metallic taste‖ in their mouths. 821 Physical Examination. The initial clinical findings are similar to those seen in either tracheal or laryngeal FB aspiration. If the object is nonobstructive and nonirritating, few or no initial symptoms may be seen. The child may have limited chest expansion, decreased vocal fremitus, atelectasis, or emphysema-like changes with resulting hyporesonance or hyperresonance. Diminished breath sounds are often found. A small object can act as a bypass valve, and homophonic wheezes can be heard. Crackles, rhonchi, and wheezes can be present if air movement is adequate. If the acute episode is missed or not appreciated, a latent period of mild ―wheezing‖ or cough may be evident. Diagnostic Studies. Clinical suspicion is the clue to this diagnosis. Inspiratory and forced expiratory chest radiographs and chest fluoroscopy are useful in identifying radiolucent FBs (Fig. 32-1). Management The patient should be referred to a pulmonary specialist for bronchoscopy. If the object is removed via bronchoscopy before permanent damage occurs, recovery is usually complete. Secondary lung infections and bronchospasms should be treated as suggested in the section on management of pneumonia in this chapter and asthma in Chapter 25. Complications If the FB is vegetable matter, vegetal or arachidic bronchitis can occur. This severe condition can be characterized by sepsis-like fever, dyspnea, and cough. If the material has been there for a long time, suppuration can occur. Lobar pneumonia, intractable wheezing, status asthmaticus can develop. Emphysema or atelectasis can also occur as the result of a large obstruction caused by a bronchial FB. Prevention. Anticipatory education regarding prevention of FB aspiration should be part of well-child supervision guidance. Parents should be cautioned about high-risk foods (e.g., whole carrots, nuts, popcorn, and hot dogs). Young children need to be supervised closely as they put small objects into their mouths as well as when they cry, shout, run, and play with food or other objects in their mouths. Nonbacterial and Bacterial Pneumonia Pneumonia is a lower respiratory tract infection associated with fever and respiratory symptoms involving the parenchyma of the lung (Gereige and Laufer, 2013). It can be lobar, interstitial, or bronchopneumonia. Lobar pneumonia involves infection of the alveolar space that results in consolidation; it is described as ―typical‖ pneumonia. Atypical pneumonia describes patterns of consolidation that are not localized. In interstitial pneumonia, cellular infiltrates attack the interstitium, which makes up the walls of the alveoli, the alveolar sacs and ducts, and the bronchioles; this type of pneumonia is typical of acute viral infections but may also be a chronic process. Viral infection affects the lung defenses by altering normal secretions, inhibiting phagocytosis, modifying the normal bacterial flora, and disrupting the epithelial layer. Many childhood viruses set the stage for secondary bacterial infection. Children with immunologic problems or chronic illnesses are prone to primary bacterial pneumonia and experience recurrent pneumonias or fail to clear the initial infection completely. Bronchial pneumonia is associated with bacterial infection 824with multiple areas of consolidation involving one or more pulmonary lobules. Pneumonitis is ageneral term used to describe lung inflammation that may or may not be associated with consolidation. Community-acquired pneumonia is acquired in the community as opposed to hospital-acquired or nosocomial pneumonia. In neonates, the risk factors for early onset pneumonia include prolonged rupture of membranes, maternal amnionitis, premature delivery, fetal tachycardia, or maternal intrapartum fever. The risk factors for late onset pneumonia include having anomalies of the airway, severe underlying disease, prolonged hospitalization, neurologic impairment, or nosocomial infection from poor hand washing or overcrowding. Risk factors for childhood pneumonia include male gender, coming from a lower socioeconomic class, poor nutrition, lack of breastfeeding, exposure to cigarette smoke (either passive or active), alcohol use, drug use, having underlying cardiopulmonary disease or neuromuscular disease, gastroesophageal reflux, tracheoesophageal fistula, or congenital and acquired immunodeficiency (Gereige and Laufer, 2013). Bacterial pneumonia occurs as a primary infection caused by organisms that spread from the nasopharynx or as a secondary complication of a viral pneumonia (Bradley et al, 2011). Primary bacterial pneumonia is less common in childhood than secondary bacterial infection after a viral infection. S. pneumoniae is the leading cause of bacterial pneumonia in all age groups except newborns (Gereige and Laufer, 2013). Certain bacterial pneumonias have a specific pattern of disease: S. pneumoniae causes a lobar pneumonia, whereas community-associated methicillin-resistant Staphylococcus aureus(MRSA) is associated with empyema and necrosis. It has been associated with influenza A; this virus may enhance the transmission of S. aureus (Geriege and Laufer, 2013). The identification of the infecting organism is difficult and, particularly in young children, leads to overuse of antibiotics. Viral pneumonia is the most common pulmonary infection, especially in children younger than 2 years old, and can result in serious illness in a young infant (Bradley et al, 2011). It often involves both the conducting airways and the alveoli. Viral infection affects the lung defenses by altering normal secretions, inhibiting phagocytosis, modifying the normal bacterial flora, and disrupting the epithelial layer. Many childhood viruses set the stage for secondary bacterial infection. Children with immunologic problems or chronic illnesses are prone to primary bacterial pneumonia and experience recurrent pneumonias or fail to clear the initial infection completely. The onset of viral pneumonia is gradual over a 1- to 2-day period of coryza, respiratory congestion, fever, cough, and increasing fretfulness (Boyer, 2009). Atypical bacterial pneumonia is caused by M. pneumoniae, Chlamydophila (formerly Chlamydia) pneumoniae, and C. trachomatis and is called walking pneumonia. M. pneumonia (or primary atypical pneumonia) is the most common cause of pneumonia in children older than 5 years through the young adult years. M. pneumoniae, an organism without a cell wall, is transmitted from one symptomatic patient to another by droplet spread. The incubation period is 2 to 3 weeks, and asymptomatic carriage after infection can last for weeks. Wheezing in a child over 5 years of age without a history of wheezing may point to an atypical pneumonia. This disease is usually mild and self-limited. C. trachomatis pneumonia is a characteristic pneumonia resulting from the transmission of C. trachomatis from the infected genital tract of the mother to the infant. It does not become apparent until the infant is 2 to 19 weeks old. Due to prenatal screening, the incidence of C. trachomatis infection of the newborn has decreased but should be considered in a mother with inadequate or no prenatal care. C. trachomatis is an organism that has many subtypes within the species. Approximately 50% of infants born to infected mothers acquire this infection, but only 5% to 20% of these infants develop C. trachomatis pneumonia with a typical onset between 1 and 3 months of age. Age influences the clinical manifestations of pneumonia and differing infectious agents cause different presentations and symptoms. Table 32-6differentiates the various forms of pneumonia commonly found in infants, children, and adolescents. Table 32-7 shows the most common infecting organisms associated with pneumonia by age. Treatment is often empirical and varies with age. TABLE 32-6 Differentiating Various Forms of Pneumonia in Infants, Young Children, and Adolescents Characteristic Bacterial Viral Mycoplasma pneumonia and Chlamydophila pneumonia Chlamydia trachomatis Common age All ages All ages >5 years old 2 to 19 weeks old (typically 1 to 3 months old) Onset Acute; gradual Acute; gradual Slow Gradual Clinical findings Depends on age; starts with URI, cough, dyspnea, tachypnea, rales, decreased breath sounds, grunting, retractions, toxic look; potential progression to severe respiratory distress Depends on age; cough, coryza, hoarseness, crackles, wheezing, stridor Persistent cough, malaise, headache Tachypnea, staccato cough, crackles, wheezing rare, 50% have signs or history of conjunctivitisCharacteristic Bacterial Viral Mycoplasma pneumonia and Chlamydophila pneumonia Chlamydia trachomatis Fever Acute onset of fever (≥102.2° F *≥39° C+) Present (less prominent) >102.2° F (>39° C) Afebrile CBC WBCs often elevated >15,000/mcl Normal or slight elevation of WBC Normal Eosinophilia in 75% of cases Organism(s) 90% caused by Streptococcus pneumoniae RSV, parainfluenza, influenza M. pneumoniae C. pneumoniae C. trachomatis Radiographic findings Lobar consolidation Transient lobar infiltrates Varies, interstitial infiltrates Hyperinflation, infiltrates Treatment Depends on bacteria and age of child; amoxicillin, penicillin, methicillin, cefuroxime, gentamicin, vancomycin Supportive care Erythromycin Azithromycin Clarithromycin Erythromycin CBC, Complete blood count; RSV, respiratory syncytial virus; URI, upper respiratory infection; WBC, white blood cell. TABLE 32-7 Age Variants in Pneumonia Microorganisms Age Viral Organisms Bacterial Organisms Neonatal Cytomegalovirus (CMV) More common Group B streptococci Gram-negative enteric bacteria Listeria Chlamydia trachomatis Uncommon organisms Streptococcus pneumoniae Group D streptococcus Anaerobes Infants Most common Respiratory syncytial virus (RSV) Parainfluenza Influenza Adenoviruses Metapneumovirus Less common S. pneumoniae Haemophilus influenzae Mycoplasma pneumonia Mycobacterium tuberculosis Bordetella pertussis Pneumocystis jiroveci Preschool children Most common RSV Parainfluenza Influenza Less common S. pneumoniae H. influenzae M. pneumoniaeAge Viral Organisms Bacterial Organisms Adenoviruses Metapneumovirus Bocavirus role is not clear M. tuberculosis Chlamydophila pneumoniae School-age children Respiratory viruses as above M. pneumoniae C. pneumoniae S. pneumoniae M. tuberculosis Adapted from Ranganathan SC, Sonnappa S: Pneumonia and other respiratory infections, Pediatr Clin North Am 56(1):140, 2009. Conjugated vaccines, such as Prevnar 13 and HIB vaccine, have decreased the incidence of these bacteria. The introduction of Prevnar 13 has decreased the incidence of pneumococcal pneumonia. Pneumococcal pneumonia occurs most commonly in the late winter and early spring after the cycle of viral URIs. Asymptomatic carriers play a more important role in dissemination of disease than sick contacts. Children younger than 4 years old suffer the highest attack rate. Clinical Findings in Infants and Young Children The hallmark of pneumonia is fever and cough in all age groups. Tachypnea and increased work of breathing may precede coughing. Cough, hypoxia, nasal flaring, rales, retractions, and rhonchus lung sounds are specific but not as sensitive for pneumonia. Viral pneumonia tends to have an insidious onset that is associated with more wheezing than what is typically noted with bacterial pneumonia. In contrast, lobar pneumonia (caused by pneumococcal pneumonia) typically presents with fever, cough, and decreased breath sounds in the area of the pneumonia. However, the child may present with a mixture of symptoms. Pneumonia can cause referred symptoms, such as abdominal pain, which may be present in a child with a diaphragmatic pneumonia or radiating neck pain that may be associated with upper lobe pneumonias. Irritation of the pleura causes the chest pain in children with pneumonia. History The following may be reported (Bradley et al, 2011; Gereige and Laufer, 2013; Iroh Tam, 2013): • Neonate • History of group B streptococcal or C. trachomatis infection in the mother 825 • Prenatal drug use or lack of prenatal care as risk factors for SBI in the neonate • With C. trachomatis, the infant is typically afebrile; prior, concurrent, or no history of inclusion conjunctivitis reported • Infant • Slower onset of respiratory symptoms, cough, wheezing, or stridor with less prominent fever suggests viral pneumonia (bacterial pneumonia is less likely in a wheezing child) • Determine mother's HIV status or infant's exposure to tuberculosis • Child and adolescent • Get immunization history and travel history of the family • Tuberculosis status • Evaluate sick contacts at home • Evaluate for possible FB aspiration • Initial history of a mild URI for a few days—similar for both bacterial and viral • Abrupt high fever with temperatures greater than 103.3° F (39.6° C), chills, cough, and dyspnea suggest bacterial pneumonia • Other manifestations include restlessness, shaking chills, apprehension, shortness of breath, malaise, and pleuritic chest pain; irritation of the pleura causes chest pain Physical Examination The health care provider needs to pay close attention to the general appearance, looking at the work of breathing, assessing for hypoxia, and evaluating tachypnea, which is considered to be the most valuable sign for ruling out pneumonia. Early onset pneumonia in the neonate presents within the first 3 days of life with: • Respiratory distress, apnea, tachycardia, poor perfusion • Tachycardia • No fever, or fever only with subtle or no physical findings Typical findings seen in all types of pneumonia include: • Nasal flaring, grunting, retractions • Tachypnea (may be the only clue), generally more than 60 breaths per minute in infants younger than 2 months old, more than 50 breaths per minute in children 2 to 82611 months old, or more than 40 breaths per minute at rest in children 1 to 5 years old • Tachycardia, air hunger, and cyanosis are significant findings• Fine crackles, dullness, diminished breath sounds • To encourage preschoolers and school-age children to breathe deeply, ask them to blow crumbled papers off your hands or use a phone application that allows them to ―blow up a balloon‖ In bacterial pneumonia: • Fever, hypoxia, lethargy • Splinting the affected side to minimize pleuritic pain or lying on the side in a fetal position helps compensate for decreased air exchange and improves ventilation • Tachypnea and retractions • Progression to delirium, circumoral cyanosis, and posturing • Presence of a pleural effusion and signs of congestive heart failure • Abdominal distention, downward displacement of the liver or spleen In viral pneumonia: • Wheezing • Downward displacement of the liver or spleen In primary atypical bacterial pneumonia (C. trachomatis): • C. trachomatis pneumonia characterized by repetitive, staccato cough with tachypnea, cervical adenopathy, crackles, and rarely wheezing • Conjunctivitis is associated with C. trachomatis in infants (Iroh Tam, 2013) Diagnostic Studies A chest x-ray should not be routinely performed in children with pneumonia (Bradley et al, 2011). However, a chest x-ray is recommended for any child older than 3 months who fails to improve after 72 hours on the standard treatment or who is being admitted. Follow-up films are not needed in patients who have an uneventful recovery. Blood cultures should not be routinely used in outpatient setting unless the child fails to improve or deteriorates on antibiotic therapy. Blood cultures should be done on children who are admitted with moderate to severe pneumonia. Sputum cultures can be used in hospitalized children who can produce sputum. Urine antigen detection tests for S. pneumonia are not recommended, because the false-positive rate is high. 827 Rapid tests for influenza and other respiratory viruses are helpful, whereas a CBC is not recommended in outpatient settings but may be helpful in inpatient settings. Acute phase reactants do not differentiate between viral and bacterial pneumonia and are not recommended for fully immunized children who are being treated as an outpatient. These tests may be useful for more seriously ill patients. Testing for M. pneumonia when the clinician is suspicious of that organism as the cause of the pneumonia can be helpful in selected situations but is not required in children with classic presentations. Differential Diagnosis The child's age and characteristic signs and symptoms as discussed previously can help distinguish between a viral and a bacterial pneumonia. The differential diagnoses to consider with pneumonia include bronchiolitis, congestive heart failure, acute bronchiectasis, FB aspiration, pulmonary abscess, parasitic pneumonia, and endotracheal tuberculosis. Right lower lobe pneumonia can present with abdominal pain and be confused with appendicitis. Right upper lobe pneumonia can often closely resemble meningitis. Management Most otherwise healthy children can be managed as outpatients. Guidelines for admission are identified in Box 32-2. Neonates must always be admitted to the hospital if diagnosed with pneumonia regardless of infecting pathogen. Young infants may also need hospitalization unless C. trachomatis is suspected. All children with pneumonia require supportive care with antipyretics, hydration, and rest. Antibiotics should be reserved for those with suspected bacterial infection only. Serious infections may require hospitalization for respiratory therapy, including humidified oxygen, pulmonary therapy, and/or intubation. Guidelines for outpatient and inpatient treatment of bacterial pneumonia by age and certain specific pathogens are as follows (Bradley et al, 2011): • Outpatient antibiotic treatment: Oral antibiotics are considered safe for most children older than 3 months with pneumonia (Bradley et al, 2011; Taketomo et al, 2014). • 2 months to 3 months old: If chlamydia is suspected, treat with oral azithromycin for 5 days or erythromycin base or ethyl succinate for 14 days (AAP, 2012). Admission may be needed depending on clinical presentation. • 3 months to 18 years old: Amoxicillin 90 mg/kg/day, divided every 12 hours for 10 days (maximum daily dose: 4000 mg/day). • If C. pneumonia or M. pneumonia (community-acquired) is suspected, azithromycin is an appropriate choice. Azithromycin 10 mg/kg/day once on day 1 (maximum dose = 500 mg) and then 5 mg/kg daily for the next 4 days (maximum dose = 250 mg). • For influenza, oseltamivir (Tamiflu) is recommended; zanamivir (Relenza) can be used in children older than 7 years. • Inpatient treatment: Testing to identify the pathogen is important for selection of appropriate antimicrobial therapy.• Neonate: Ampicillin and cefotaxime, ceftriaxone, or gentamicin. • Ampicillin or penicillin G in a fully-immunized infant or school-age child with community-acquired pneumonia unless there is a high incidence of S. pneumoniae. • Empiric therapy with a third-generation cephalosporin (ceftriaxone or cefotaxime) if there are high rates of penicillin-resistant S. pneumoniae. • The addition of macrolide to a beta-lactam therapy, if M. pneumonia and C. pneumonia are present or considered likely to be present. • Vancomycin or clindamycin in addition to beta-lactam therapy if S. aureus is strongly considered, keeping in mind that communityacquired MRSA may require more than 10 days of therapy (Bradley et al, 2011). Parental education about medication administration, hydration, fever control, and worrisome signs and symptoms is important. In addition, the child should be seen for follow-up at the conclusion of antibiotic treatment or sooner if there is no improvement or worsening of 828symptoms. Children with recurrent pneumonias should be referred for further pulmonary evaluation. Prognosis By the second to third day of treatment, auscultation should reveal a change in respiratory sounds as the infection begins to consolidate. Increased fremitus, tubular breath sounds, and the disappearance of crackles may be noted. Most children have an uneventful recovery, but it is important to inform parents that their child's cough can last for several weeks. Routine rechecks with chest x-rays are not recommended. If pneumonia recurs or persists for longer than 1 month, the child needs further evaluation for underlying immunodeficiency disease. Complications Complications associated with community-acquired pneumonia can involve other systems and include meningitis, CNS abscess, endocarditis, pericarditis, osteomyelitis, and/or septic arthritis. Pulmonary complications include empyema (which is more common in staphylococcal and GABHS infections), pneumothorax, bronchopleural fistula, and/or lung abscess. Scarring of the airways and lung tissue can cause dilated bronchi, which results in bronchiectasis. M. pneumoniae can spread to the blood, CNS, heart, skin, or joints. A child with sickle cell disease and pneumonia caused by M. pneumoniae has more severe pulmonary disease than the average child does. Prevention Identify and treat pregnant women with C. trachomatis. Universal vaccination against influenza, HIB, and pneumococcal infection is essential. Current guidelines limit the use of palivizumab (Synagis) prophylaxis (see Chapter 24). Diarrheal Illnesses Due to Common Bacterial or Viral Pathogens Etiology Incubatio n Period Signs and Symptoms Duration of Illness Route of Transmissio n Laboratory Testing Treatment and Complications* Campylobacter jejuni 2 to 5 days, but can be long er Diarrhea (foul smelling), cramps, fever, nausea and vomiting; diarrhea may be bloody in neonates Occurs in warm weather months 2 to 10 days Raw and undercook ed poultry, unpasteuri zed milk, contaminat ed water; low inoculum dose produces infection Routine stool culture; Campylobacte rrequires special media and incubation temperature; positive gross blood, leukocytes; CBC: ↑ WBCs Rehydration is the mainstay. Azithromycin and erythromycin shorten the duration of the illness when given early, and usually eradicates the organism from stool within 2 to 3 days. Clostridium difficile Unknown Variety of symptoms and severity are seen: mild to explosive diarrhea, bloody stools, abdominal pain, fever, nausea, vomiting Mild to moderate illness is characterized by watery diarrhea, low-grade fever, and mild abdominal pain During or after severa l weeks of antibi otic use; can occur witho ut being associ Acquired from the environme nt or from stool of other colonized or infected people by the fecaloral route Stool cultures; enzyme immunoassay for toxin A, or A and B; positive gross blood, leukocytes; CBC: ↑ WBCs; ESR normal Discontinue current antibiotic (any antibiotic, but notably ampicillin, clindamycin, second- and thirdgeneration cephalosporins). Fluids and electrolyte replacement are usually sufficient. If antibiotic is still needed or illness is severe, treat with oral metronidazole (drug of choice in children) orEtiology Incubatio n Period Signs and Symptoms Duration of Illness Route of Transmissio n Laboratory Testing Treatment and Complications* ated with such treatm ent vancomycin for 7 to 10 days. Supplement with probiotics. Lactobacillus GG, Saccharomyce s boulardii are recommended (Jones, 2010; Shane, 2010). Complications include pseudomembranous colitis, toxic megacolon, colonic perforation, relapse, intractable proctitis, death in debilitated children. Enterohemorrhagic Escheric hia coli(EHEC) including E. coli O157:H7 and other Shiga toxin– producing E. coli(STEC) 1 to 8 days Severe diarrhea that is often bloody, abdominal pain and vomiting Usually little or no fever More common in children <4 years old 5 to 10 days Undercooked beef, especially hamburger , unpasteuri zed milk and juice, raw fruits, vegetables (e.g., sprouts, spinach, lettuce), salami (rarely) Contaminate d water; petting zoos Stool culture; E. coliO157:H7 requires special media to grow. If E. coliO157:H7 is suspected, specific testing must be requested. Shiga toxin testing may be done using commercial kits; positive isolates should be forwarded to public health laboratories for confirmation and serotyping. Stool grossly positive for blood. Supportive care: Monitor CBC, platelets, and kidney function closely. E. coli O157:H7 infection is also associated with HUS, which can cause lifelong complications. Studies indicate that antibiotics may promote the development of HUS. Enterotoxigenic E. coli(ETEC) and enteroadherent E. coli (frequent cause of traveler's diarrhea) 1 to 3 days Watery diarrhea, abdominal cramps, some vomiting; often cause of mild traveler's diarrhea 3 to >7 days Water or food contaminat ed with human feces Stool culture. ETEC requires special laboratory techniques for identification. If suspected, must request specific testing. Supportive care: Antibiotics are rarely needed except in severe cases. Recommended antibiotics include TMP-SMX and quinolones. See www.cdc.gov/t ravel. Listeria monocytogenes Variable, rangi ng from 1 day to more than 3 week Rare, but serious Fever, muscle aches, and nausea or diarrhea Pregnant women may have mild flulike illness, and infection can lead to premature delivery or stillbirth Older adults or Variable Thrives in salty and acidic conditions, such as fresh soft cheeses, ready-toeat deli meats, hot dogs; also unpasteuri zed milk, Blood or cerebrospinal fluid cultures. Asymptomatic fecal carriage occurs; therefore, stool culture usually not helpful. Antibody to listeriolysin O may be helpful to identify outbreak retrospectively. Initial therapy with IV ampicillin and an aminoglycoside usually gentamicin, recommended for severe infections.Etiology Incubatio n Period Signs and Symptoms Duration of Illness Route of Transmissio n Laboratory Testing Treatment and Complications* s immunocompro mised patients may have bacteremia or meningitis Infants infected from mother at risk for sepsis or meningitis inadequate ly pasteurize d milk; multiplies at low temperatur es, even in properly refrigerate d foods Adenovirus, enteric 3 to 10 days Children >4 years old Variable Fecal-oral, throughout year; can remain viable on inanimate objects Stool specimen for adenovirus antigen via rapid commercial immunoassay techniques or per electron microscopy Supportive care: Monitor intake and hydration status Preventive care: Good hand washing and diapering precaution Norovirus 12 to 48 hour s Abrupt-onset watery diarrhea, nausea, vomiting, abdominal cramps 24 to 60 hours Often associ ated with closed venue s (child care center s, cruise ships) Fecal-oral; contaminat ed food (ice, shellfish, ready-toeat foods [e.g., salads, bakery products], or water) No commercial assay available; CDC can support laboratory evaluation or state and local health department laboratories can perform RT-PCR assays. Supportive care: May need to treat dehydration and/or electrolyte imbalance. Preventive care: Hand hygiene, clean surfaces and food preparation areas; no swimming in recreational venues for 2 weeks after symptoms resolve. Rotavirus 1 to 3 days; prev alent durin g cool er mont hs in temp erate clim ates Acute-onset fever, vomiting, and watery diarrhea occur 2 to 4 days later in children <5 years old, especially those between 3 to 24 months old 3 to 8 days Fecal-oral; viable on inanimate objects; rarely contaminat ed water or food Enzyme immunoassay and latex agglutination assays for group A rotavirus antigen; virus can be found by electron microscopy and specific nucleic acid amplification methods. Supportive care: May need to correct dehydration and electrolyte imbalances. Oral IG has been used in those immunocompromis ed. Preventive care: Rotavirus vaccine; hygiene and diapering precautions in day care facilities. Salmonella spp. 1 to 3 days Diarrhea, fever, abdominal cramps, rebound tenderness, vomiting. S. typhi and S. paratyphi produc 4 to 7 days Contaminate d eggs, poultry, unpasteuri zed milk or juice, cheese, Routine stool cultures; positive leukocytes and gross blood. CBC: WBC can be slightly ↑ with left shift, ↓, or Supportive care: Only consider antibiotics (other than for S. typhi or S. paratyphi) for infants <3 monthsEtiology Incubatio n Period Signs and Symptoms Duration of Illness Route of Transmissio n Laboratory Testing Treatment and Complications* e typhoid with insidious onset characterized by fever, headache, constipation, malaise, chills, and myalgia; diarrhea is uncommon, and vomiting is not usually severe contaminat ed raw fruits and vegetables (alfalfa sprouts, melons) S. typhiepide mics are often related to fecal contaminat ion of water supplies or streetvended foods normal. old, those with chronic GI disease, malignant neoplasm, hemoglobinopathie s, HIV, other immunosuppressive illnesses or therapies. If indicated, consider ampicillin or amoxicillin, azithromycin, or TMP-SMX; if resistance shown to any of those, use IM ceftriaxone, cefotaxime; or azithromycin or quinolones. A vaccine exists for S. typhi in certain cases. Shigella spp. Varies from 1 to 7 days, but typic ally is 1 to 3 days Abdominal cramps, fever, and diarrhea; Stools may contain blood and mucus Seen most commonly in those 6 months old to 3 years old 4 to 7 days Food or water contaminat ed with human fecal material Usually person-toperson spread, fecal-oral transmissi on Ready-to-eat foods touched by infected food workers (e.g., raw vegetables, salads, sandwiche s) Routine stool cultures; gross blood, leukocytes. CBC: normal or slightly ↑ WBCs with left shift Supportive care: If antibiotics indicated (severe disease, dysentery, immunocompromis ed), test first for susceptibility. Oral ampicillin (amoxicillin less so) or TMP-SMX recommended in the United States; for organism resistance, use IM ceftriaxone for 2 to 5 days; PO ciprofloxacin; azithromycin (oral cephalosporins not useful). If child is at risk of malnutrition, supplement with vitamin A (200,000 international units). No swimming in recreational pools/slides for 1 week after symptoms resolve. Yersinia enterocolyticaand Y. pseudotuberculosis Typically 4 to 6 days with a rang e of 1 to 14 Appendicitis-like symptoms (diarrhea and vomiting, fever, and RLQ pain) occur primarily in older children and young adults May have a scarlatiniform rash or erythema nodosum with Y. 1 to 3 weeks, usuall y selflimitin g Undercooked pork, unpasteuri zed milk, tofu, contaminat ed water Infection has occurred in infants whose caregivers Stool, vomitus, or blood culture. Yersinia requir es special medium to grow. If suspected, must request specific testing. Serology is available in research and reference Supportive care: If septicemia or other invasive disease occurs, antibiotic therapy with gentamicin or cefotaxime (doxycycline and ciprofloxacin also effective) afterEtiology Incubatio n Period Signs and Symptoms Duration of Illness Route of Transmissio n Laboratory Testing Treatment and Complications* days pseudotuberculo sis Seen in all ages handled chitterlings laboratories. susceptibility testing is done. Pyloric Stenosis Pyloric stenosis is characterized by hypertrophied pyloric muscle, causing a narrowing of the pyloric sphincter. Pyloric stenosis occurs in 3 per 1000 live births, with a fourfold increase in males compared with females (Hunter and Liacourus, 2011b). It tends to be familial and is seen more commonly in Caucasian first-born males. Clinical Findings History. • Regurgitation and non-projectile vomiting during the first few weeks of life • Projectile vomiting beginning at 2 to 3 weeks old • Insatiable appetite with weight loss, dehydration, and constipation • An association of pyloric stenosis with the administration of erythromycin in the first 2 weeks of life has been demonstrated Physical Examination. • Weight loss • Nonbilious vomitus that can contain blood • A distinct ―olive‖ mass that is often palpated in the epigastrium to the right of midline • Reverse peristalsis visualized across the abdomen Diagnostic Studies. Ultrasound, with measurement of the pyloric muscle thickness, is used in most centers. An upper gastrointestinal series demonstrates a ―string sign,‖ indicating a fine, elongated pyloric canal may be required if ultrasound is unavailable or inconclusive. Management and Prognosis Surgical intervention (pyloromyotomy) is indicated after correction of fluid and electrolyte imbalance. Vomiting can continue for a few days after surgery, although it is not as significant as it was preoperatively; feedings should be introduced gradually. The prognosis is excellent. Hirschsprung Disease (Congenital Aganglionic Megacolon) Hirschsprung disease is an absence of ganglion cells in the bowel wall, most often in the rectosigmoid region, resulting in a portion of the colon having no motility. This disorder occurs in 1 in 5000 births. It is the most common cause of neonatal obstruction of the colon and accounts for approximately 33% of all neonatal obstructions. The disease is familial, affects males four times more commonly than females, and is common in children with trisomy 21. Additional anomalies are sometimes present (Hunter and Liacourus, 2011b). Clinical Findings • Failure to pass meconium within the first 48 hours of life • Failure to thrive, poor feeding • Chronic constipation, vomiting, abdominal obstruction • Diarrhea, explosive bowel movements, or flatus • Down syndrome Diagnostic Studies. Radiographs indicate dilated loops of bowel (Fig. 39-16). A biopsy determines the absence of ganglion cells. Differential Diagnosis The differential diagnosis includes acquired functional megacolon, colonic inertia, chronic idiopathic constipation, obstipation, small left colon syndrome, meconium plug syndrome, and ileal atresia with microcolon.Management Surgical resection of the affected bowel is indicated, with or without a colostomy. Intestinal Parasites Various protozoa and helminths can invade the GI tract and cause disease. In developed countries, such infestations are usually by protozoa. Endemic areas of developing countries are subject to more significant morbidity and mortality from parasitic infestations. All can multiply within the human body, are associated with diarrheal symptoms, and are spread by fecal contamination due to poor water and sewage disposal practices. Cysts of these parasites are often resistant to chlorine. Helminths are worms; nematodes (roundworms), cestodes (tapeworms), and trematodes (flatworms) that most commonly reside in the human intestines but do not multiply there. Fecal-oral contact with eggs or cysts excreted from the initial vector via ingestion of contaminated food or water is one route of infestation. Some helminths (hookworms and whipworms) release larvae into the soil; humans become infected when they walk barefoot on contaminated soil and the skin is penetrated by the larvae. These larvae then travel to the lungs and intestines. Eggs can also be excreted in the stool; poor sanitary disposal of human waste into soils affords the further potential for ingestion via contamination of food and water. They are found worldwide, principally in tropical and subtropical developing countries. In industrialized countries, infestation is found in those who travel to endemic areas, in the immunocompromised, and immigrants from endemic areas. Their insidious nature causes chronic health and nutritional problems that can impair physical and mental growth of children. Enterobius vermicularis (pinworm), Ascaris lumbricoides (roundworm), and Taenia (tapeworm) are some of the more common intestinal parasites that affect the pediatric population (Harhay et al, 2010). Clinical Findings, Management, and Differential Diagnosis See Tables 33-14 and 33-15 for clinical findings and management. The differential diagnosis includes all other causes of infectious and noninfectious diarrhea. TABLE 33-14 Intestinal Illnesses Due to More Common Parasites (Protozoa and Helminths) Etiology Incubation Period Signs and Symptoms Duration of Illness Route of Transmission Laboratory Testing Treatment* Cryptosporidium parvum 3 to 14 days Diarrhea (usually watery), stomach cramps, upset stomach, bloating, slight fever, anorexia, weight loss, flatulence, nausea, vomiting, fatigue Self-limiting, usually lasts 6 to 14 days Fecal-oral route; from uncooked food or food contaminate d by an ill food handler after cooking; drinking water (collects on water filters and membranes that cannot be disinfected); reservoirs include cattle, sheep, goats, birds, reptiles, young animals Request specific testing of the stool for Cryptosporidium using antigendetection tests. May need to examine water or food. Supportive care, selflimited. If severe, or individual immunocompromise d consider paromomycin for 7 days. For children 1 to 11 years old, consider nitazoxanide for 3 days.Etiology Incubation Period Signs and Symptoms Duration of Illness Route of Transmission Laboratory Testing Treatment* Cyclospora cayetanensis Usually 7 days, but can range from 2 to 14 days Diarrhea (usually watery), loss of appetite, substantial loss of weight, stomach cramps, nausea, vomiting, fatigue, myalgia, lowgrade fevers May remit and relapse over weeks to month s Fecal-oral from sewage or nontreated water; food (various types of fresh produce [imported berries, lettuce]) Request specific examination of the stool for Cyclospora. May need to examine water or food. TMP-SMX for 7 to 10 days. Entamoeba histolytica Commonly 2 to 4 weeks (known to also range from days, months , to years); fecaloral transmi ssion Can be asymptomatic with nonspecific complaints of diarrhea, lower abdominal pain In invasive disease (amebic colitis) symptoms of increasing diarrhea, bloody diarrhea, lower abdominal pain, tenesmus, weight loss progress over a 1- to 3-week period; occasional fever Advanced disease hepatomegaly , liver tenderness Weeks to years, depen ding on respon se to treatm ent; no drug is comple tely effecti ve Spread by fecaloral route Stool examination for trophozoites or cysts; PCR, isoenzyme analysis, monoclonal antibody–based antigen; enzyme immunoassay; ultrasounds and CT scans to identify suspected liver abscess or other extraintestinal infection. Asymptomatic cyst excreters: Luminal amebicide (iodoquinol, paromomycin, diloxanide). Mild to moderate or severe involvement/liver abscesses: metronidazole or tinidazole followed by luminal amebicide. Follow-up stool examination after treatment. Perform stool examinations on household members or other suspected contacts. Do not treat with corticosteroids or antimotility drugs. Complications include liver abscess, ameboma. Giardia intestinalis 3 weeks Can include bouts of watery diarrhea; abdominal pain, greasy, foul-smelling stools; bloody diarrhea (rare); flatulence; abdominal distention; anorexia; weight loss; Pending effecti ve treatm ent Fecal-oral or contaminate d food or water. Water can be contaminate d by Giardia fr om dogs, cats, beavers, and other Stool specimens for trophozoites or cysts using staining methods; antigens using enzyme immunoassay; PCR techniques. Increased sensitivity by obtaining three or more specimens every other day and by rapid examination of stool (can be Correct for any dehydration or electrolyte imbalance. Tinidazole, metronidazole, nitazoxanide drugs of choice. Albendazole, mebendazole effective also in children with fewer side effects. Consult for those immunocompromise d. Contact local health departmentsEtiology Incubation Period Signs and Symptoms Duration of Illness Route of Transmission Laboratory Testing Treatment* FTT; anemia; asymptomatic infection common animals. placed in a fixative). in cases of outbreaks. Infected individuals should not use recreational water sources for swimming until 2 weeks after symptoms resolve. Filtration, boiling, chemical disinfection may be required for drinking water. Some infections are self-limited and treatment is not required. Dehydration and electrolyte abnormalities can occur and should be corrected. Complications: Debilitating disease leading to malabsorption; anorexia; weight loss; FTT. Ancylostoma duodenale(hookwor m) 5 to 8 weeks for eggs to appear in feces; 4 to 12 weeks for onset of sympto ms Often asymptomatic or stinging/burni ng sensation in feet followed by pruritus, papulovesicul ar rash (lasting up to 2 weeks), pharyngeal itching, hoarseness, nausea, vomiting As migrates through lungs: Mild cough, pneumonitis Chronic infestation: Anemia, edema, growth delays, slowed development and cognition in children 5 year unless treated Larvae in fecescontaminate d soil penetrate skin (travel to lungs and settle in the intestines) or are directly ingested from contaminate d food or water, including human milk. CBC shows hypochromic microcytic anemia, eosinophilia; hypoproteinemia. Stool microscopic examination for ova. Albendazole, pyrantel pamoate; repeat stool examination in 2 weeks recommended. Iron and nutritional supplementation if indicated; severe cases require blood transfusions. Complications: Delayed growth, developmental/ment al status delays in children. Enterobius vermicularis(pinwor m) 1 to 2 months , or longer from Perirectal and/or vaginal pruritus; nervous irritability, Reinfection comm on in childre Ingested eggs from soil, water contaminati on, or direct 1 cm long white, threadlike worms can be visualized at anus during night after child has been Mebendazole, pyrantel pamoate or albendazole and repeated in 2 weeks; also treat family members; vaginitis is self-limiting.Etiology Incubation Period Signs and Symptoms Duration of Illness Route of Transmission Laboratory Testing Treatment* ingesti on to migrati on to periana l area hyperactivity, insomnia; urethritis, vaginitis, salpingitis, and pelvic peritonitis have been reported n fecal-oral route from fomites on bedding, clothing, toys, baths; person-toperson. Female lays eggs in perianal area and dies; ingested eggs hatch, become larvae in small intestine and migrate to rectum. asleep for 2 to 3 hours. Microscopic examination: Use transparent adhesive tape applied to anus to collect any eggs or pinworms present on three consecutive nights or mornings before child arises. Direct stool examination usually not productive. Easily spread among family members, in day care settings, and institutions (up to 50% infestation rates in these populations). Preventive: Morning baths, change bedding, hand hygiene, clip fingernails, avoid scratching perianal region, avoid nail biting. Day care precautions include hand hygiene, proper handling of underwear and diapers. Ascaris lumbricoides(round worm) 8 weeks from egg ingesti on to adult egglaying capacit y Weight loss, malnutrition; worms can be seen in vomitus and stools; can cause cough, fever, chest discomfort if pass through lungs (not a common occurrence) Children can have large worm burdens Stressful conditions (fever, illness) and some anthelmintic drugs can cause adults to migrate 12 to 18 month s withou t treatm ent Fecal-oral from ingestion of eggs from contaminate d food (fruit, vegetables) or soil (where incubation occurs; adult worms live in small intestine and eggs are excreted in feces). Larvae migrate from intestines via portal blood to liver and lungs, ascend through tracheobron chial tree to pharynx, to intestines again to develop into adults. Found in areas where human feces are used for Stool/vomitus/nares: Worms seen via microscopy. CBC: Marked eosinophilia. Have laboratory check for all concurrent worm infestations in order to treat all worms appropriately. Albendazole, mebendazole, ivermectin; surgical intervention if necessary. Complications: Impaired nutritional status of children and growth; bowel or biliary obstruction, peritonitis, obstruction of common bile duct (biliary colic, cholangitis, pancreatitis); Löffler syndrome due to allergic response as larvae migrate to the lungs. Reinfection common. Globally, most common human intestinal nematode.Etiology Incubation Period Signs and Symptoms Duration of Illness Route of Transmission Laboratory Testing Treatment* fertilizer. Taenia (tapeworm) (T. saginata[beef]; T. solium[pork]) 2 to 3 months after larvae ingeste d to feces excreti on Worm(s) may be seen in perianal region May be asymptomatic or have abdominal pain, nausea, diarrhea, excessive appetite Several years before cystice rcosis sympto ms eviden t Fecal-oral from ingestion of water or food contaminate d with eggs or from ingested cysts or larvae in inadequatel y cooked pork or beef. Stool microscopy: ova seen. Praziquantel, niclosamide, nitazoxanide. Complications: Systemic cysticercosis from T. solium(viscera, brain, muscle invasion with possible seizures). Trichuris trichiura(whipworm) 12 weeks Asymptomatic unless infestation is heavy; abdominal pain, tenesmus, bloody diarrhea with mucus; can mimic IBD; growth retardation Fecal-oral from contaminate d soil (where eggs incubate), water, and/or food (embeds in mucosal lining of large intestines). Not spread person to person. Stool microscopy or concentration techniques. Mebendazole, albendazole, ivermectin for 3 days; can reexamine stools after 2 weeks to ensure resolution. Complications: Chronic colitis, rectal prolapse, compromised nutritional status, growth retardation. *See Table 33-15 for dosages. CBC, Complete blood count; CT, computed tomography; FTT, failure to thrive; IBD, inflammatory bowel disease; PCR, polymerase chain reaction; TMPSMX, trimethoprim-sulfamethoxazole. TABLE 33-15 Medications for Treatment of Parasite Infestations* Drug Dosage Albendazole (Albenza): Take with food. The tablet may be crushed or chewed and swallowed with a drink of water. Ascariasis: 1 year old: 200 mg once; >2 years old: 400 mg once Taenia solium: 15 mg/kg/day in 2 doses × 8 to 30 days; can be repeated as necessary (maximum 400 mg per dose) Mebendazole (Vermox): Tablet may be crushed, mixed with food, swallowed whole, or chewed. Pinworms: 100 mg once; may need to repeat in 2 weeks Whipworms, roundworms, and hookworms: 100 mg twice daily for 3 days Toxocariasis: 100-200 mg twice daily for 5 daysDrug Dosage Diloxanide furoate (Furamide): Give with meals. 20 mg/kg/day in 3 doses × 10 days (maximum 500 mg per dose) Ivermectin (Stromectal): Take on an empty stomach. All ages: 150-200 mcg/kg/dose once Iodoquinol (Yodoxin): Administer after meals, tablets may be crushed and mixed with applesauce or chocolate syrup. 30-40 mg/kg/day in 3 doses × 20 days (maximum 2 g) Adults: 650 mg three times daily × 20 days Metronidazole (Flagyl) 35-50 mg/kg/day in 3 doses × 7 to 10 days (maximum 500-750 mg per dose) Giardia lamblia: 15 mg/kg/day in 3 doses × 5 days (maximum 250 mg per dose) Nitazoxanide (Alinia): Administer with food, shake suspension well prior to use. 1 to 3 years old: 100 mg in 2 doses × 3 days 4 to 11 years old: 200 mg in 2 doses × 3 days 11 years old to adult: 500 mg twice daily × 3 days Paromomycin (Humatin): Administer with or without meals, protect from moisture. 25-35 mg/kg/day in 3 doses × 7 days Praziquantel (Biltricide): Administer tablets with water during meals; do not chew due to bitter taste. Tapeworm: 5-10 mg/kg once Liver Fluke: Up to 75 mg/kg in 3 doses in 1 day Pyrantel pamoate (Pamix, Pin-X): Over the counter. May be mixed with milk or fruit juice. Shake suspension well. 11 mg/kg daily (maximum 1 g) × 3 days Use with caution in children <2 years old Tinidazole (Tindamax) Giardia lamblia: 50 mg/kg once (maximum 2 g) *Medication choices and dosages are dependent upon child's condition at diagnosis and parasite. Consult CDC or latest recommendations for treatment regimes. Data from Schleiss MR, Chen SF: Principles of antiparasitic therapy. In Kliegman RM, Behrman RE, Jenson HB, et al: Nelson textbook of pediatrics, ed 19, Philadelphia, 2011, Elsevier. Patient and Family Education Most parasitic infestations can be prevented by good hand washing and good sanitation. The following preventive measures are recommended: • Travelers to developing countries need to eat only foods that can be peeled or have been cooked. Ice, ―washed‖ foods, and tap water can be contaminated. Bottled or treated water is advised for drinking and brushing teeth. Shoes should be worn when walking on potentially contaminated soil.888 • G. lamblia: Encourage good hand hygiene. Prevent contamination of water sources. Treat questionable water with iodine, boiling for 20 minutes, or use commercial filters to filter contaminated water. Exclude symptomatic children and staff from school and day care until asymptomatic. • E. vermicularis: Avoid scratching. Wash sheets and clothing in hot water and detergent. • A. lumbricoides: Appropriate food preparation is necessary to prevent infection. When human feces are used for fertilizer, thoroughly cook or soak fruit and vegetables in diluted iodine solution before consuming. Periodic, empiric treatment of children may prevent nutritional and cognitive deficits in endemic areas. • Taenia: Avoid raw or undercooked beef or pork. Gastroesophageal Reflux Disease Gastroesophageal reflux refers to the passage of gastric contents into the esophagus from the stomach through the LES. It is a normal physiologic process that occurs several times a day in healthy infants, children, and adults. “GERD is present when the reflux causes troublesome symptoms and or complications” (Vandenplas et al, 2009, p 499). GERD is the most common esophageal disorder in children (Khan and Orenstein, 2011b). The etiology of GERD is unclear and probably multifactorial. Inappropriate relaxation of the LES with failure to prevent gastric acid reflux into the esophagus, prolonged esophageal clearance of the gastric refluxate, and impaired esophageal mucosal barrier function are the likely causes of most GERD (Loots et al, 2014). LES function usually is influenced by intraabdominal pressure, hormones, neurologic control, and age. Young infants have increased intraabdominal pressure because of their inability to sit upright. They can also regurgitate when they cough, cry, or strain. In healthy infants, regurgitation is highest in the first month of life (73%) and decreases to 50% by the fifth month of life. During the first 2 months of life, 20% of infants regurgitate more than four times per day. After 1 year old, less than 4% of infants regurgitate daily and nearly all resolve by 2 years old. Weight gain is less in infants who regurgitate more than four times per day and breastfed babies regurgitate less than formula-fed babies (Khan and Orenstein, 2011b). Alterations in swallowing, pharyngeal coordination, esophageal motility, and delayed gastric emptying are also potential factors related to GERD. Increased muscle tone, chronic supine positioning, and altered GI motility exacerbate GERD. Helicobacter pylori has been associated with GERD. Children with H. pylori are about six times more likely to develop GERD than non–H. pylori-positive children. H. pylori has not been found in infants younger than 1 year old (Polat and Polat, 2012). The American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) states that 10% of infants younger than 1 year old with regurgitation develop significant complications (GERD) (AAO-HNS, 2011). Risk factors include prematurity, neurologic impairment, obesity, CF, hiatal hernia, and family history of GERD. Clinical Findings Common signs and symptoms by age that should lead the clinician to suspect GERD are found in Table 33-4; 845although, according to the guidelines, there is no symptom or symptom complex that is diagnostic of GERD or predicts response to therapy. In older children and adolescents, history and physical examination may be sufficient to diagnose GERD. The most common symptom is ―heartburn.‖ Recurrent regurgitation with or without vomiting, weight loss or poor weight gain, ruminative behavior, hematemesis, dysphagia, and respiratory disorders such as, wheezing, stridor, cough, apnea, hoarseness, and recurrent pneumonia are also associated with GERD. TABLE 33-4 Symptoms and Signs that May Be Associated with Gastroesophageal Reflux Symptoms and Signs that Vary by Age Symptoms for All Children Signs for All Children Infancy: Regurgitation; signs of esophagitis (irritability, arching, choking, gagging, feeding aversion); FTT. Usually symptoms resolve between 12 and 24 months of age. Obstructive apnea, stridor, lower airway disease by which reflux complicates a primary airway disease (e.g., bronchopulmonary dysplasia). Otitis media, sinusitis, lymphoid hyperplasia, hoarseness, vocal cord nodules, laryngeal edema. Child and adolescent: Regurgitation during preschool years, complaints of abdominal and chest pain, neck contortions (arching, turning of head), asthma, sinusitis, laryngitis Recurrent regurgitation with/without vomiting Ruminative behavior Heartburn or chest pain Hematemesis Dysphagia, odynophagia Respiratory disorders, such as wheezing, stridor, cough, hoarseness, persistent throat clearing or cough Halitosis Esophagitis Esophageal stricture Barrett esophagus Laryngeal/pharyngeal inflammation Recurrent pneumonia Anemia Dental erosion Apnea spells Apparent lifethreatening events Weight loss or poor weight gainFTT, Failure to thrive. Adapted from Vandenplas Y, Rudolph C, Di Lorenzo C, et al: Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN), J Pediatr Gastroenterol Nutr 49(4):498–547, 2009, p 519. History Box 33-4 summarizes the history for GERD that should be collected according to the national guidelines. Warning signs that merit urgent investigation of vomiting are found in Box 33-5. Box 33-4 H is to ry f or th e Chi ld w i th Susp ect ed Ga s tro es opha g ea l R ef lu x Di s ea se Feeding and Dietary History • Amount/frequency (overfeeding) • Preparation of formula • Observe the child during a feeding (clinician) • Recent changes in feeding type or technique • Position during feeding, burping technique and frequency Behavior during Feeding • Choking, gagging, coughing, arching, discomfort, refusal Pattern of Vomiting • Frequency and amount, pain, forceful • Blood or bile • Associated fever, lethargy, diarrhea Medical History • Prematurity and newborn screen results • Growth and development, previous weight and height gain (growth charts) • Past surgery, hospitalizations • Recurrent illnesses, especially croup, pneumonia, asthma • Symptoms of hoarseness, fussiness, hiccups, apnea • Other chronic conditions • Medications: Current, recent, prescription, nonprescription Family Psychosocial History • Sources of stress and/or postpartum depression • Maternal or paternal drug use Family Medical History • Significant illnesses • Family history of gastrointestinal (GI) disorders • Family history of atopy Box 33-5 W a rnin g Sign al s R equi rin g U rgent I nv es ti ga t ion in Inf an t s w i th R egu rg it a ti on o r V o mi ti ng • Bilious vomiting • Gastrointestinal (GI) bleeding, hematemesis, hematochezia • Consistently forceful vomiting or onset of vomiting after 6 months old • Failure to thrive (FTT) • Recurrent respiratory infections • Feeding problems (uncoordinated swallow, choking or cough associated with feeding) • Diarrhea or constipation • Fever and/or lethargy• Hepatosplenomegaly • Bulging fontanelles, macrocephaly, or microcephaly • Seizures • Abdominal tenderness or distension • Documented or suspected genetic/metabolic syndrome Physical Examination • Review of height, weight, and head circumference • Signs of FTT • Torticollis: Neck arching • Hoarseness • Anemia • Tooth erosion resulting from destruction of enamel by gastric acids caused by frequent vomiting • Rash, recurrent diarrhea, persistent vomiting, or early-morning vomiting (symptoms of other primary disease with GERD as a secondary problem) Diagnostic Studies In most infants with vomiting and in older children with regurgitation and heartburn, a history and physical examination are sufficient to reliably diagnose GERD, recognize 846complications, and initiate treatment. An empiric trial of acid suppression with a PPI for 4 weeks may be used as a diagnostic test in older children and adolescents but is not recommended in infants and young children. Nonradiologic diagnostic tests as indicated: • CBC with differential to rule out anemia and infection • UA and urine culture • Stool for occult blood • Testing for H. pylori The following specialized tests may be obtained following consultation with a physician or a pediatric gastroenterologist. • Esophageal pH monitoring has been the gold standard to diagnose reflux. However, the presence of reflux may not correlate with the severity of illness, and some gastric contents may not be acidic. Transnasal pH placement may be uncomfortable, decrease appetite and activity, and thus underestimate the true incidence of reflux episodes. Typically, patients are asked to discontinue H2 blockers for 72 hours before the test and PPIs for 1 week before the study. • Multichannel intraluminal impedance (MII) measures episodes of reflux independent of the pH of the fluid. It is especially useful for making a diagnosis in children with respiratory events related to reflux, because it can measure multiple indices, such as heart rate, oxygenation, sleep state, and apnea episodes. It can also measure the height of refluxed material and the content and direction of the reflux (liquid, air, or both). It is preferred by many gastroenterologists because it can measure acid and nonacid reflux (50% of reflux in infants is nonacidic). Cost, time to interpret results, and lack of consensus about norms for frequency or length of nonacidic reflux events are disadvantages to this study (Vandenplas et al, 2009). • Wireless pH monitoring is also available. A pH probe is placed transorally, temporarily attached to the esophageal mucosa where it is programmed to record events for 48 hours. The capsule typically sloughs in about 5 days. Failure to attach, chest pain, feeling of foreign body, and premature detachment are negative aspects of this technology. • Endoscopy to obtain a biopsy can help determine severity of reflux esophagitis. It can rule out esophagitis and other pathologic conditions if deemed necessary. It may also be used to re-dilate strictures. • Barium upper GI series should only be used if obstruction or an anatomic abnormality of the upper GI tract is suspected. • Radionuclide scan with scintiscan and esophageal and gastric ultrasonography studies are not recommended for routine evaluation of GERD. • Gastric emptying scan can be used to evaluate for delayed gastric motility associated with GER. • A video swallow study may be necessary if recurrent respiratory infection, persistent cough, or feeding refusal (or difficulty) is present to evaluate for effective esophageal swallow and to rule out aspiration. Differential Diagnosis The clinician should also consider other causes of vomiting as found in Table 33-2. Management TABLE 33-5 Management Strategies for Infants and Children with Gastroesophageal RefluxPopulation Diagnostic Tests Management Strategies Infant with uncomplicated recurrent regurgitation (GER) None needed Provide parental education and reassurance. In formula-fed babies, a thickened formula may reduce over-regurgitation and vomiting but does not reduce the reflux itself. Infants with recurrent vomiting and poor weight gain (GERD) Diet history, UA, CBC, serum electrolytes, BUN, serum creatinine Other tests as indicated For breastfed infants, continue to breastfeed. For formula-fed babies, 2-week trial of extensively hydrolyzed formula or amino acid– based formula to exclude CMA. Increase caloric density. Thicken formula if needed. Educate regarding formula intake needed to sustain normal weight gain. Refer to pediatric gastroenterologist if management fails to improve symptoms and weight gain. Infants with unexplained crying and/or distressed behavior Evaluate for CMA, neurologic disorders, constipation, infection (especially UTIs) Empiric trial with extensively hydrolyzed protein formula or amino acid–based formula. No evidence to support the empiric use of acid suppression for the treatment of irritable infants. However, if irritability persists and no condition other than GERD remains, then continued support of parents with the anticipation of improvement over time; workup to establish the relationship of reflux to feeding or to diagnose esophagitis; or trial of antisecretory therapy, although there is a potential risk for adverse effects. Clinical improvement following empiric therapy may result in spontaneous symptom resolution or placebo response. Child older than 18 months old with chronic regurgitation or vomiting Consider diagnosis other than GERD; testing may include upper GI endoscopy, esophageal pH/MII, and barium upper GI series Treatment depends on diagnosis. Heartburn in older children and adolescents No further studies needed if problem is episodic and not severe On-demand therapy with buffering agents, sodium alginate, or H2RA may be used for occasional symptoms. For chronic heartburn, lifestyle changes, such as diet change, weight loss, smoking avoidance, sleeping position, no late-night eating, and a 2-week trial with a PPI may help. PPI can be continued for up to 3 months if symptoms resolve. Persistent heartburn after that time should be referred to a pediatric gastroenterologist. Reflux esophagitis— endoscopically diagnosed No further studies needed PPI for 3 months is initial therapy. Trial of tapering the dose and then withdrawal of PPI. Chronic relapsing esophagitis may be the diagnosis if PPI cannot be withdrawn and may involve long-term therapy with PPI or antireflux surgery. BUN, Blood urea nitrogen; CBC, complete blood count; CMA, cow's-milk allergy; GER, gastroesophageal reflux; GERD, gastroesophageal reflux disease; GI, gastrointestinal; H2RA,histamine 2 receptor antagonist; MII, multichannel intraluminal impedance; PPI, proton pump inhibitor; UA, urinalysis; UTI, urinary tract infection. Adapted from Vandenplas Y, Rudolph C, Di Lorenzo C, et al: Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN), J Pediatr Gastroenterol Nutr 49(4):498–547, 2009. Pharmacologic Acid-suppression agents are the mainstays of treatment. These pharmacologic agents include H2RAs, PPIs, and buffering agents (Table 33- 6). TABLE 33-6 Common Medications Used to Treat Gastroesophageal Reflux DiseaseMedication Pediatric Dosage Histamine 2 Receptor Antagonists Cimetidine Infants: 10-20 mg/kg/day divided doses every 6 to 12 hours Children: 20-40 mg/kg/day in divided doses every 6 hours Adult dose: 300 mg/dose PO qid OR 400 mg/dose PO bid OR 800 mg/dose PO qhs Famotidine (Pepcid) Infants: 1 to 3 months old: 0.5 mg/kg/dose once daily for up to 8 weeks Infants >3 months old to 1 year old: 0.5 mg/kg/dose twice daily for up to 8 weeks Children and adolescents: Initially 0.25 mg/kg/dose every 12 hours (maximum dose: 20 mg/dose) Nizatidine (Axid) Infants 6 months old to children 11 years old: 5-10 mg/kg/day divided twice daily Children 12 years+: 150 mg once daily Ranitidine (Zantac) Infants >1 month, children, and adolescents <16 years old: 4-8 mg/kg/day divided twice daily (maximum dose: 300 mg) Adolescents >16 years old: 150 mg twice daily or 300 mg once HS Proton Pump Inhibitors Lansoprazole (Prevacid) Children 1 to 11 years old: <30 kg: 15 mg once daily for up to 12 weeks >30 kg: 30 mg once daily for up to 12 weeks Omeprazole (Prilosec) Children > 1 year: 5 to 10 kg: 5 mg once daily for up to 12 weeks 10 to 20 kg: 10 mg once daily for up to 12 weeks >20 kg: 20 mg once daily for up to 12 weeks Rabeprazole (Aciphex) Children 1 to 11 years old: <15 kg: 5 mg once daily for <12 weeks >15 kg: 10 mg once daily for <12 weeks Adult dose: 20 mg once daily for <12 weeks Pantoprazole (Protonix) Infants and children <5 years old: 1.2 mg/kg/day once daily for 4 weeks Children 5 to 11 years old: 20-40 mg once daily for up to 8 weeks Children and adolescents 12 to 16 years old: 20 or 40 mg once daily for up to 8 weeks Esomeprazole (Nexium) Infants: 3 to 5 kg: 2.5 mg once daily for up to 8 weeks 5 to 7.5 kg: 5 mg once daily for up to 8 weeks >7.5 kg: 10 mg once daily for up to 8 weeks Children 1 to 11 years old: <20 kg 10 mg once daily for up to 8 weeks >20 kg: 10-20 mg once daily for up to 8 weeks Children >12 years old: 20-40 mg once daily for up to 8 weeks Contents of capsule may be mixed with 1 tablespoon of applesauce for easier swallowing, if needed.Medication Pediatric Dosage Cytoprotective Agent Sucralfate (Carafate) 40-80 mg/kg/day divided every 6 hours Adult dose 250 mg divided every 6 hours Take on an empty stomach 1 hour before meal and HS Data from Engorn B, Flerage J: The Harriet Lane handbook: a manual for pediatric house officer, ed 20, Philadelphia, 2015, Elsevier; Khan S, Orenstein S: Gastroesophageal reflux disease. In Kliegman RM, Stanton BF, St. Geme JW, et al: Nelson textbook of pediatrics, ed 19, Philadelphia, 2011, Elsevier; Lightdale JR, Gremse DA, Section on Gastroenterology, Hepatology, and Nutrition: Gastroesophageal reflux: management guidance for the pediatrician, Pediatrics 131(5):e1684–e1685, 2013. PPIs are superior to H2RAs in relieving symptoms and promoting mucosal healing and do not result in tolerance as do H2RAs. However H2RAs and buffering agents have a rapid onset of action and are useful in on-demand treatment (Vandenplas et al, 2009). There is insufficient evidence to justify the routine use of prokinetic agents such as cisapride, metoclopramide, domperidone, bethanechol, erythromycin, or baclofen for GERD. Because safe and convenient alternatives are available that are more acceptable to patients, chronic antacid therapy is generally not recommended for patients with GERD (Vandenplas et al, 2009). Nutrition Feeding techniques, volumes, and frequency of feeding should be normalized. A trial of extensively hydrolyzed protein formula may be used for 2 to 4 weeks in formula-fed infants with vomiting. Thickening agents for formula (1 tablespoon rice cereal/ounce formula) reduce regurgitation but not significantly (Hegar et al, 2008). An increase in caloric density may be necessary in infants with FTT (poor weight gain or weight loss). In older children and adolescents, there is no evidence to support specific dietary restrictions to decrease symptoms; however, avoiding eating 847less than 2 hours before bedtime may be helpful. Obesity is related to GERD, so weight management could be helpful. Lifestyle Because prone positioning is associated with increased risk of sudden infant death syndrome (SIDS), supine positioning during sleep in infants is recommended. Positioning infants upright may worsen reflux. There may be some benefit in older children to left-side positioning during sleep or elevation of the head of the bed (elevate the head of the bed and don't add pillows because it may increase abdominal flexion and compression). 848 Surgical Antireflux surgery strategies, such as fundoplication, are used for management of cases that have not responded to less invasive strategies, have life-threatening complications, or will have long-term dependence on medical therapy in which compliance or patient preference precludes ongoing use. However, the surgery is not necessarily curative. For example, in one study of fundoplication in children with cystic fibrosis (CF), 12% had repeat surgery, 48% had recurrent GERD symptoms, and only 28% discontinued GERD medications (Vandenplas et al, 2009). Now that PPI therapy is so successful, fundoplication may become less common (Khan and Orenstein, 2011b). Complications Complications include chronic cough, FTT, irritability, and malnutrition. Esophageal injury secondary to reflux results in bleeding, stricture formation, and Barrett esophagus. GERD is circumstantially associated with significant asthma, recurrent pneumonia, or laryngeal disorders. In the majority of infants with apnea or apparent life-threatening event, GERD is not the cause. However, in the rare case where a relationship is suspected, pH monitoring in combination with polysomnographic recording and precise, synchronous symptom recording may aid in establishing cause and effect (Vandenplas et al, 2009). Red flags in infants are bilious vomiting and/or hematemesis (see Box 33-5). Patient and Family Education • Assure parents of infants that regurgitation is usually self-limited and symptoms improve as the child grows. Parental education and reassurance are recommended for infants with uncomplicated regurgitation. Remind parents that GERD may temporarily worsen during illness. • Review medication information, including dosages and side effects. Urinary Tract Infection and PyelonephritisThere are three kinds of UTI in children: (1) asymptomatic bacteriuria, (2) cystitis, and (3) pyelonephritis. Young children may have limited or unusual symptoms; therefore, a high degree of suspicion must be maintained to diagnose UTI. Inflammation and infection can occur at any point in the urinary tract, so a UTI must be identified according to location. Asymptomatic bacteriuria is bacteria in the urine without other symptoms, is benign, and does not cause renal injury. Cystitis is an infection of the bladder that produces lower tract symptoms but does not cause fever or renal injury (Elder, 2011d). Pyelonephritis is the most severe type of UTI involving the renal parenchyma or kidneys and must be readily identified and treated because of the potential irreversible renal damage that can occur. Clinical signs thought to be consistent with pyelonephritis include fever, irritability, and vomiting in an infant, and urinary symptoms associated with fever, bacteriuria, vomiting, and renal tenderness in older children. UTIs are the most common cause of serious bacterial infection in infants younger than 24 months old with fever without a focus (Elder, 2011d). A complicated UTI is defined as a UTI with fever, toxicity, and dehydration or a UTI occurring in a child younger than 3 to 6 months old. The UTI may be classified based on its association with other structural or functional abnormality, such as VUR, obstruction, dysfunctional voiding, or pregnancy. Additionally, a UTI must be identified as a first occurrence, recurrent (within 2 weeks with the same organism or any reinfection with a different organism), or chronic (ongoing, unresolved, often caused by a structural abnormality or resistant organism). Finally, age and gender of the pediatric patient are important factors in determining the method of evaluation and the course of treatment. The organism most commonly associated with UTI is Escherichia coli (70%), although other organisms (such as, Enterobacter, Klebsiella, Pseudomonas, and Proteus) can cause infection. UTI secondary to group B streptococcus is more common in neonates. Several factors are believed to contribute to the etiology of UTIs. Most UTIs are thought to be ascending (i.e., the infection begins with colonization of the urethral area and ascends the urinary tract). If the infection progresses to the kidney, intrarenal reflux deep into the kidneys can lead to scarring. However, the most important risk factor for the development of pyelonephritis in children is VUR, which can be detected in 10% to 45% of young children who have symptomatic UTIs. Furthermore, reflux of infected urine from the bladder increases the risk of pyelonephritis. This damage to the kidney occurs in the compound papillae, which have wide and gaping 916openings allowing intrarenal reflux. The compound papillae are located in the upper and lower poles of the kidney, which is the usual site of scarring. Simple conical papillae have angled, slit-like openings that resist intrarenal reflux (Fig. 35-2). Host resistance factors and bacterial virulence factors are also important in the etiology of UTIs. Host resistance factors include the presence of a structural abnormality or dysplasia (such as, VUR, obstruction, or any other anatomic defect) or the presence of functional abnormalities (such as, dysfunctional voiding or constipation). Other factors affecting resistance include female gender (having a short urethra), poor hygiene, irritation, sexual activity or sexual abuse, and pinworms. Several bacterial factors are known, but the two most important ones are adherence and virulence of the bacteria. Bacteria that have fimbriae or pili are able to anchor or adhere to the surface of the bladder mucosa. This adherence allows the bacteria to resist the bladder's defensive cleansing flow of urine and causes tissue inflammation and cell damage. Adherence may also play a role in bacteria ascending the urinary tract. Virulence refers to the toxicity of substances released by bacteria. The greater the virulence, the greater the damage to the urinary tract. Both of these factors enhance colonization of the urinary tract and aid in the persistence and effect of the bacteria. The risk of UTI in infants 2 to 24 months old is about 5%. The incidence in females is more than twice that of males (2.27%); uncircumcised boys have a rate 4 to 20 times greater than circumcised boys (AAP Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, 2011). There is a greater frequency in premature and low-birthweight infants. Females older than 12 months old have 2.1% prevalence; after the first year of life, it is also more common to find a UTI in females than in males with an overall incidence of 1% to 3% to in girls and 1% in boys (Elder, 2011d). The incidence of UTI is often increased in adolescent girls as they become sexually active. Recurrence is common, often within the first year after the initial infection. Clinical Findings History The following information should be obtained: • Family history of VUR, recurrent UTI, or other kidney problems • Prenatally diagnosed renal abnormality • Previous infection: Request records from the evaluation of past infections and diagnostic studies performed • Circumcision • Risk factors for infants 2 to 24 months old with no other source of infection (AAP Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, 2011) • Female—white race, age younger than 12 months old, temperature 39° C or higher, fever for 2 days or more • Male—nonblack race, temperature 39° C or higher, fever for more than 24 hours• Hygiene habits: Wiping front to back • Voiding patterns: Frequency, abnormal stream, complete emptying, dribbling, and enuresis • Constipation, perianal itching (pinworms) • Irritants, such as nylon underwear or clothing (spandex, tight pants or shorts that rub); bubble bath or sitting in soapy bath water • High BP • Sexual activity, masturbation, or sexual abuse • Other infection: Pinworms, diaper rash Physical Examination See Table 35-2 for age-related symptoms. • General appearance (toxic appearing?) • Vital signs: Temperature, BP • Growth parameters: Growth may be decreased with chronic UTI or renal insufficiency, especially in infants • Flank pain or tenderness in the costovertebral angle • Abdominal examination: Suprapubic tenderness, bladder distention or a flank mass (obstructive signs), mass from fecal impaction • Genitalia: Vaginal erythema, edema, irritation, or discharge; labial adhesions; uncircumcised male, urethral ballooning; weak, dribbling, threadlike stream • Neurologic examination (if voiding is dysfunctional): Perineal sensation, lower extremity reflexes, sacral dimpling, or cutaneous abnormality TABLE 35-2 Clinical Findings of Urinary Tract Infection in Children of Various Ages Neonates Infants Toddlers and Preschoolers School-Age Children and Adolescents Jaundice Malaise, irritability Altered voiding pattern “Classic dysuria” with frequency, urgency, and discomfort Hypothermia Difficulty feeding Malodor Failure to thrive (FTT) Poor weight gain Abdominal/flank pain* Malodor Sepsis Fever* Enuresis Enuresis Vomiting or diarrhea Vomiting or diarrhea Vomiting or diarrhea* Abdominal/flank pain* Cyanosis Malodor Malaise Fever/chills* Abdominal distention Dribbling Fever* Vomiting or diarrhea*Neonates Infants Toddlers and Preschoolers School-Age Children and Adolescents Lethargy Abdominal pain/colic Diaper rash Malaise *Findings increase likelihood of pyelonephritis. 917 Diagnostic Studies The method used to collect urine has an effect on the interpretation of results. It is acceptable to collect urine for UA only from a non–toilettrained child by using a sterile, adhesive bag carefully placed over well-cleaned genitals. If the bagged urine results in a positive leukocyte esterase or nitrite test, a child younger than 24 months old has risk factors, or the patient is symptomatic, additional urine should be collected by sterile catheterization or suprapubic aspiration. Older children, who can void on command, should be able to obtain a clean-catch void. Having the female child sit backward on the toilet separates the labia and decreases contamination. See the Diagnostic Studies section earlier in this chapter for other pertinent information. • Urine culture by standard culture methods is essential to confirm the diagnosis. If the culture shows greater than 100,000 colonies of a single pathogen in a clean catch urine specimen, greater than 50,000 in a catheterized or suprapubic specimen, or if there are 10,000 colonies of a single pathogen and the child is symptomatic, the child is considered to have a UTI (Elder, 2011d; Shaw, 2015) • UA should be used only to raise or lower suspicion. Suspicious findings include foul odor, cloudiness, nitrites, leukocytes, alkaline pH, proteinuria, hematuria, pyuria, and bacteriuria. • Nitrite chemical tests are reliable on urine specimens when gram-negative bacteria are present and when the urine has been in the bladder for 4 hours or longer. False-positive results are rare, whereas false-negative results are common. • Leukocyte esterase chemical tests detect pyuria, but pyuria may arise from causes other than UTI. • Microscopic evaluation of uncentrifuged urine may be helpful if bacteria are seen. • Gram stain may be helpful if bacteria are identified. • Bacterial identification and determination of sensitivities are necessary in patients who appear toxic or could have pyelonephritis, have relapses or recurrent UTI, or are nonresponsive to medication. • Complete blood count (CBC) (elevated WBC count), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), BUN, and creatinine should be done if the child is younger than 1 year old, appears ill, or if pyelonephritis is suspected. • Serum procalcitonin level of more than 0.5 ng/mL is an accurate and reliable biologic marker for renal involvement during a febrile UTI, pyelonephritis, and with renal scarring, so it may be useful in the clinical diagnosis and treatment of UTIs (Leroy et al, 2011) • Blood culture should be done if sepsis is suspected (see Chapter 24). Differential Diagnosis The differential diagnosis includes urethritis, vaginitis, viral cystitis, foreign body, sexual abuse, dysfunctional voiding, appendicitis, pelvic abscess, and pelvic inflammatory disease. Any child who has acute fever without a focus, FTT, chronic diarrhea, or recurrent abdominal pain should be evaluated for UTI. Management Goals of treatment are to quickly identify the extent and level of infection; to treat appropriately to eradicate infection; to provide symptomatic relief; to find and correct anatomic or functional abnormalities; and to prevent recurrence and new or progressive renal damage (AAP Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, 2011). When deciding on a treatment plan, the child's age, gender, symptoms, the suspected location of the UTI and antibiotic 918resistance patterns in the community must be considered. Figure 35-3 outlines treatment of UTI in the child. Infants 2 to 24 Months Old To diagnose UTI, the child should have both a UA suggesting infection (positive leukocyte and/or nitrite tests) and urine culture from a sterile catheterization or suprapubic aspiration (SPA) with at least 50,000 cfu/mL. Risk factors have been identified to help steer management. See the AAP Guideline (AAP Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, 2011). Asymptomatic Bacteriuria If there is an absence of leukocytes on UA, no treatment is indicated. Uncomplicated Cystitis There is no agreement on the most effective antimicrobial agent or dosage for treating a UTI (Fitzgerald et al, 2012). Short-term antibiotics of 3 to 5 days may be as effective in treating non-febrile bladder infectionsas standard 7- to 10-day dosing with no increased risk of recurrence (Elder, 2011d). Children 2 to 24 months old and febrile children should have 7 to 14 days of antibiotics. Specific recommendations for the febrile child can be found in the AAP Guideline (AAP Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, 2011). Choice of antibiotic should be made based on regional antibiotic resistance patterns and culture and sensitivity results. Recommended oral medications include the following (Elder, 2011d; Lee et al, 2015): • Trimethoprim-sulfamethoxazole (TMP-SMX): More than 2 months old—8 to 12 mg/kg TMP component in two divided doses; adolescents, 160 mg TMP component every 12 hours. • Amoxicillin: Younger than 3 months old—20 to 30 mg/kg/day in two divided doses every 12 hours; older than 3 months old—25 to 50 mg/kg/day in two divided doses; adolescents, 250 to 500 mg every 8 hours or 875 mg every 12 hours. • Amoxicillin clavulanate (doses for amoxicillin component): Younger than 3 months old—30 mg/kg/day in two divided doses; older than 3 months old—20 to 45 mg/kg/day in two or three divided doses; adolescents—250 to 500 mg every 8 hours or 875 mg every 12 hours. • Cephalexin: 50 to 100 mg/kg/day divided in four doses and given every 6 hours (maximum dose of 4 g/day). • Cefixime: Older than 6 months old—16 mg/kg/day divided every 12 hours for first day, then 8 mg/kg/day divided every 12 hours to complete 13-day treatment; adolescents—400 mg every 12 to 24 hours. • Cefpodoxime proxetil: 2 months to 12 years old—10 mg/kg/day divided every 12 hours (maximum dose of 400 mg/day); adolescents—200 to 800 mg/day divided every 12 hours (maximum dose of 800 mg/day). • Ciprofloxacin extended release: Older than 18 years old—500 mg once a day for 3 days. • Nitrofurantoin: Older than 1 month old—5 to 7 mg/kg/day divided every 6 hours (maximum 400 mg/24 hours); adolescents—50 to 100 mg/dose every 6 hours (macrocrystals) or 100 mg twice a day (dual release). • Recurrent UTI: Further evaluation required (ultrasound, if not done previously and VCUG). Use of prophylactic antibiotics (Box 35-1) is controversial • Acute pyelonephritis: Oral therapy is equally as effective in treating pyelonephritis and preventing kidney damage as parenteral antimicrobials (Engorn and Flerlage, 2015). • Hospitalization is required if severity of symptoms warrants—dehydrated, vomiting, or not drinking. Children 1 month old and younger should be admitted and provided a parenteral regimen. • Young children with uncomplicated pyelonephritis (well hydrated, no vomiting, no abdominal pain) can be effectively treated with cefixime, ceftibuten, or amoxicillin clavulanate. • Adolescents with uncomplicated pyelonephritis can be treated with either amoxicillin clavulanate (875/125 mg twice a day) or ciprofloxacin (500 mg twice a day or extended release 1000 mg once a day). • Follow-up urine culture should be done 48 to 72 hours after initiating treatment if symptoms persist or organism resistance is found in the community. • If the culture is not sterile or if no clinical improvement is seen, antibiotic change should be based on sensitivity report received. Urine should be sent for bacterial identification and sensitivity studies now if not performed initially and an alternative broad-spectrum antibiotic should be used pending those results. Culture should again be repeated after 48 to 72 hours if response to therapy limited. • Follow-up cultures are not routinely needed; however, when obtained for recurrent problems should be obtained 3 to 7 days after finishing antibiotic treatment. • Repeat urine culture should be done with any fever, illness, dysuria, or frequency. • Phenazopyridine may be given at 12 mg/kg/day for 6- to 12-year-olds and 200 mg for those older than 12 years old, three times a day for dysuria. • Radiologic workup (Table 35-3) is recommended to identify any structural or functional abnormality of the urinary tract and any renal scarring or damage. • Children younger than 2 years old with the first UTI should have a renal and bladder ultrasound as soon as the urine is sterile or when the prescribed antibiotic has been completed. Additionally, all children with fever or diagnosed with pyonephritis or with recurrent UTIs should have a renal and bladder ultrasound. VCUG does not need to be done routinely with first febrile UTI. However, if ultrasound reveals hydronephrosis, scarring, or other atypical or concerning findings, DMSA scan should be completed. If DMSA scan is unavailable, VCUG can be utilized (Elder, 2011d).• DMSA scan may be recommended for children with a febrile UTI or when a diagnosis of pyelonephritis is uncertain. If the DMSA scan is abnormal, then a VCUG is recommended (Elder, 2011d). Patient and Family Education, Prevention, and Prognosis The following should be discussed with parents and/or patients: • Clear explanation of the cause, potential complications, and overall treatment plan, including both shortand long-term plans. • Frequent and complete voiding and increased quantities of fluids, especially water. Sometimes scheduled voiding times, voiding with knees spread apart, or double voiding (voiding and then immediately attempting to void again) can be helpful. • Proper hygiene and avoiding irritants, such as bubble baths and perfumed soaps. Avoid wearing tight pants, especially spandex pants. Wear cotton underwear. Treat perineal inflammation to help prevent UTI. • Treatment of constipation; pinworms. • Sexually active females should be encouraged to drink water before intercourse and void immediately afterward. • Decrease intake of bladder irritants, such as the ―four Cs‖ (caffeine, carbonated beverages, chocolate, citrus), aspartame (NutraSweet), alcohol, and spicy foods. • Importance of prompt medical attention with recurrence of fever and/or duration of fever for more than 48 hours, especially if younger than 24 months old. • Cranberry juice is considered helpful in preventing the adherence of E. coli in the urethra but must be consumed in large quantities in order to be effective (see Chapter 43). According to Fitzgerald and colleagues (2012), ―The relationship between UTI, renal scarring, and VUR is unclear, as is the progression of uncomplicated UTI to pyelonephritis and subsequent damage to the kidneys.‖ Major risk factors for renal damage include delay in treatment of pyelonephritis, younger than 1 year old, anatomic or neurogenic obstruction, severe reflux, dysplasia, and multiple infections. The same acute inflammatory process responsible for eradication of bacteria is also responsible for damage to renal tissue and subsequent scarring. Enuresis Enuresis is defined as voluntary or involuntary urination into bed or clothes at an age when toilet training should be complete. Children who have never established control have primary enuresis. Secondary enuresis is present when children have been dry for more than 6 to 12 months and begin wetting. Nocturnal enuresis is incontinence during sleep. If a child has normal daytime elimination with no concerns, then nighttime wetting is called monosymptomatic nocturnal enuresis (MNE). More commonly, children with nocturnal enuresis have BBD symptoms during the day as well; this type of nocturnal enuresis is non-monosymptomatic nocturnal enuresis (NMNE). Diurnal enuresis, daytime wetting, occurs during waking hours. Diagnosing enuresis can be a challenge. According to the ICCS, using ICD-10 and DSM-V, a diagnosis of enuresis requires a minimum age of 5 years old, and one episode a month for a duration of 3 months. The ICCS goes on to state that enuresis is frequent if it occurs four or more times a week and infrequent if it occurs four or less times a month (Austin et al, 2014). It is important to remember that the age at which urinary continence is normally achieved varies greatly and thus children should be evaluated on a case-by-case basis, taking into account the child and family dynamics and developmental stages and the amount of duress the issue is causing. Regardless of the numbers, if the parent or child asks for help, they should receive it. The cause of enuresis varies among children and can be difficult to determine. A number of factors have been found to be associated with enuresis, including the following: • Constipation: It cannot be overemphasized how important it is to determine if constipation or impaction exists before treating nocturnal enuresis. • Familial disposition: Even if there is a presumed genetic predisposition based on parental history, many of these children have constipation; and if that is treated, they have improvement. • Neurologic developmental delay • Behavioral comorbidities (e.g., externalizing behaviors): There appears to be a strong association between enuresis (especially daytime enuresis) and attention-deficit/hyperactivity disorder (ADHD) (von Gontard et al, 2011). • Functional small bladder capacity: In some children, bladder capacity appears normal during the day but is functionally reduced at night (Godbole et al, 2011). • Sleep disorders: Obstructive sleep apnea and disordered sleep patterns are associated with increased incidence of nocturnal enuresis (Godbole et al, 2011). • Stress and family disruptions: Some examples are a divorce, move, or a new family member. • Polyuria: This can be caused by nocturnal drinking as well as caffeine intake (Godbole et al, 2011).• Inappropriate toilet training: This is especially common when parents are overly demanding or punitive of the child. Clinical Findings The goals of assessment are to (1) determine if there are comorbid or underlying conditions that require pediatric urology referral and (2) establish the best approach to treating this particular child's condition. History It is essential to gather the most honest nighttime and daytime history of elimination habits possible, both urine and bowel. Parents should be asked about the following: • Voiding characteristics: • Urgency, dysuria, or dribbling • Are there voiding or stooling postponement behaviors? • Number of voids per day: Is nocturia present? 230 • Cluster voiding: For example, is the child waiting until after school? • Frequency of wetting—day and night • Type of urinary stream • These findings warrant referral to a pediatric urologist (Nevéus et al, 2010): • Weak or interrupted urinary stream • Need to use abdominal pressure to urinate • Daytime incontinence and nocturnal enuresis combined • Fluid intake, how much and when • UTI • History of enuresis, treatment, and age of resolution for other family members, including parents • History of toilet training: What age was toilet training begun? How was it handled? Was the child ever dry? For how long? • Effect of enuresis on child and parents • Manner in which family deals with the enuresis: For example, is the child punished? Who changes the bed? Any previous medical treatment? • Bowel patterns: What is the frequency? Is there constipation? Is there fecal incontinence? What is the quality of stool (see Box 12-1)? • Sleep patterns: Look for indications of obstructive sleep-disordered breathing or apnea. Reassure parents that deep sleep is not a cause for nocturnal enuresis. • General health: • Prenatal and perinatal history • Is child tired? Has child lost weight? Does child have excessive thirst or hunger (e.g., diabetes)? • Has the child been diagnosed with a neuropsychological condition (e.g., ADHD)? • Presence of other behavior problems • Changes in the home, family, or school environment: Be sure to determine if the enuresis was present before any disruptive changes occurred. Physical Examination The physical examination includes the following: • Assess the external genitalia for signs of irritation, infection, labial fusion, and/or meatal stenosis. • Check for fecal impaction. • Examine the abdomen for masses, especially at the suprapubic midline and in the left lower quadrant. • Examine the lower back for dimples and hair tufts. • Assess for neurologic function and DTR. Diagnostic Studies A urinalysis is recommended in all children with enuresis. A culture should be done if there are clinical symptoms to warrant it. More sophisticated testing is usually not necessary. Differential Diagnosis The differential diagnosis includes daytime extraordinary urinary frequency (pollakiuria), which is a benign condition of excessive urination (more than 8 to 12 times per day, often as frequent as every 15 to 30 minutes) seen in previously toilet-trained children who do not need to void at night. Pollakiuria has no known cause, but it may be associated with viral cystitis or urethritis, stress, and hypercalciuria. Although considered self-limited because it does not typically respond to medication, pollakiuria can persist for months or even years; however, it typically lasts about 6 months (Farber, 2013).Organic causes of enuresis must be identified. The most common organic cause is UTI that may be related to BBD. Following is a list of other organic causes to consider, and worsening incontinence, development of neurologic signs (e.g., weakness in legs), and increased urine volumes or dilution warrant referral to specialists for further evaluation. • Diabetes mellitus • Diabetes insipidus • Sickle cell disease, in which treatment by means of forced fluids may lead to increased urine output • Chronic renal failure, in which the kidneys are unable to concentrate urine • Structural anomalies, such as ectopic ureter (constant leakage is noted) or a vesicovaginal fistula • Neurologic abnormalities, including neurogenic bladder • Hypercalciuria • Obstructive uropathy other than that due to BBD • Vaginitis • Sleep apnea Management The goals of treatment are to establish normal bladder function and prevent both physical and emotional or psychological complications. A thorough examination to distinguish between organic and nonorganic causes is the first crucial step. Intervention is then based on the underlying cause and involves behavioral modification, medication, treatment of comorbid or organic conditions, or a combination of these modalities. Treatment of daytime urinary dysfunction and constipation should be done before treating nocturnal enuresis (Van de Walle et al, 2012). Referral to a pediatric urology specialist may be necessary. Outcomes of treatment are categorized as: • No response: Less than 50% decrease in enuresis • Partial response: 50% to 99% reduction • Complete response: 100% reduction Over the long term, outcomes include: • Relapse: More than one symptom relapse per month • Continued success: No return of symptoms in 6 months • Complete success: No return of symptoms after 2 years Because functional enuresis is largely self-limited, there is consensus to delay aggressive treatment until the child is 6 to 8 years old. Treatment strategies for children 6 years old or older include the following: • Urotherapy: A non-pharmacologic, nonsurgical intervention, urotherapy is basic to treatment of enuresis (see Box 12-4). Urotherapy increases daytime urination by 231establishing a regular voiding schedule—not waiting until the micturition urge is felt. It also limits nighttime urine production by regulating fluid intake. The goal is for the bladder to be able to hold urine produced overnight. Children should void before going to bed and again immediately upon waking in the morning. Proper posture while urinating is important to help the child be more sensitive to cues of a full bladder and to control urination. This approach has been used effectively for children with hyperactive bladders and may make medication unnecessary for many children. Urotherapy also involves aggressive treatment of constipation. • Enuresis alarms: According to the ICCS, use of enuresis alarms and desmopressin medication are equivalent first-line therapies that can be used to treat enuresis (Nevéus et al, 2010); the decision to use an alarm should be made after discussion with the child and family and be based on their preference. Alarm therapy seems to be more effective in children with “decreased maximal voided volumes” (Maternik et al, 2015). A review of the research literature indicates that long-term alarm therapy is more effective than desmopressin for treatment of primary MNE (Perrin et al, 2013), and an enuresis alarm is considered firstline treatment when conditions such as diabetes, kidney disease, or urogenital malformations have been ruled out (Nevéus, 2011). Use of an alarm requires commitment and effort on the part of parents and extensive support from the primary care provider. • Drug therapy: Drug therapy (see Table 12-4 for dosing and comments) can be combined with urotherapy and/or alarm therapy, but it is not curative. It usually has high initial success rates. Unfortunately, drug therapy can be expensive, and high relapse rates can occur when the drug is discontinued. When the wetting recurs, it can be very upsetting to the child, which is a factor that needs to be considered when prescribing. However, it can be very useful for overnight stays (e.g., camp) when staying dry is important to the child. Desmopressin has an antidiuretic effect and appears to be most effective in children with large nocturnal urine production and normal nocturnal bladder capacity. Its effect is immediate and it can be taken only on nights that the child wants to be sure to stay dry (Nevéus et al, 2010). Desmopressin is available in three forms: nasal spray, oral tablets, or oral lyophilisate preparation (MELT) (sublingual administration). Nasal spray has led to hyponatremia, has a black box warning from the FDA, and is not recommended for routine use (Robson, 2009). Patients should be cautioned to avoid high fluid intake with the oral medication, to be sure that the correct dosage is given, and to discontinue the medication if headache, nausea, or vomiting occurs (Robson et al, 2007; Van de Walle et al, 2010). Use of the MELTpreparation reduces fluid intake and has been shown in one study to reduce bed-wetting by a factor of two over use of the tablet form (Juul et al, 2013). Desmopressin may be more effective when combined with urotherapy. Other drugs are not recommended as first-line treatment. These include anticholinergics (antimuscarinic drugs [also used for treatment of overactive bladder]: oxybutynin, tolterodine, and solifenacin), which can cause constipation and could complicate the problem; botulinum toxin type A (BtA); and imipramine, which should only be used as third-line therapy at tertiary care facilities, if at all, due to its cardiotoxic side effects. Sacral nerve stimulation for children with severe voiding dysfunction that has not responded to aggressive urotherapy and medical interventions is currently being studied. Complications Enuresis contributes to poor self-esteem and disrupted family interactions and threatens the child's ability to 232establish strong peer relationships. Parents of children with nocturnal and diurnal enuresis rate those children as having more problem behaviors than do parents of children without enuresis; these parents also rate their own stress level as higher (De Bruyne et al, 2009); children with enuresis are at risk for child abuse. Effective treatment improves behavior and self-concept, suggesting that enuresis precedes behavior problems. Patient and Family Education Supportive, proactive education of parents and positive reinforcement of children's efforts can help prevent enuresis. For 3- to 5-year-old children, a nonjudgmental attitude of ―benign neglect‖ in the face of accidents is the best approach. For older children with enuresis, aggressive, long-term interventions are appropriate; wetting is a common phenomenon, and parents should be reassured that it rarely indicates disease. Dealing with a child who wets frequently can be frustrating, however, and parents need to know that the provider is committed to working closely with them until the child is dry. Nephritis and Glomerulonephritis Nephritis is a noninfectious, inflammatory response of the kidneys characterized by varied degrees of hypertension, edema, proteinuria, and hematuria that can be either microscopic or macroscopic with dysmorphic RBCs and casts. Nephritis is classified as acute, intermittent, or chronic. Primary GN occurs when the original and predominant structure impaired is the glomerulus. Secondary GN occurs when renal involvement is secondary to systemic disease (e.g., SLE, HSP, primary vasculitis, Goodpasture syndrome, or drug hypersensitivity reactions). Involvement can be in the glomerulus or the interstitium and either localized in one part of the kidney or generalized throughout. GN refers to inflammation primarily in the glomeruli; interstitial nephritis refers to inflammation in the interstitium primarily caused by drug reactions. PSGN is the classic form of GN. Acute nephritis most commonly occurs as PSGN, which is characterized by a history of streptococcal infection within the prior 2 weeks and an acute onset of edema, oliguria, hypertension, and gross hematuria. Consider an alternative diagnosis if the following findings are present: nephrotic levels of protein, lack of evidence for a post-infection mechanism, rapidly deteriorating renal function, or clinical or laboratory findings suggesting other forms of GN (e.g., rash, positive ANA). Intermittent gross hematuria and proteinuria syndromes include the following: • IgA nephropathy, or Berger disease, is the most common chronic GN in children of European Asian descent and is uncommon in African Americans; it has a 2 : 1 male preponderance. It is an immunologic entity causing recurrent gross and microscopic hematuria and often proteinuria. It is present in about one third of persons biopsied for persistent microscopic hematuria. It is often precipitated by viral infections or strenuous exercise, and each episode lasts less than 72 hours. BP is normal, no edema is present, and C3 is normal. Definitive diagnosis is made by biopsy. The prognosis is good in the absence of elevated serum creatinine or nephrotic-range proteinuria, although progression to chronic renal insufficiency can occur. • Hereditary or familial nephritis involves many disorders, but the best known is Alport syndrome. More common and severe in males, with onset before 15 years old in 75% of children, this condition is inherited as an X-linked dominant trait. The initial manifestation is isolated, persistent, microscopic hematuria with intermittent macrohematuria and variable proteinuria, occurring with an upper respiratory infection or exercise. Laboratory abnormalities are variable; biopsy verifies the diagnosis. Extrarenal abnormalities, including neurogenic deafness, ocular abnormalities, and macrothrombocytopenia, are often found. Vision and hearing screening are essential with referral for any abnormalities. Severe forms of the disease can lead to end-stage renal disease, which is often heralded by hypotension. • Familial or benign recurrent nephritis, also known as thin-basement-membrane disease, is a disorder inherited as an autosomal dominant trait with unknown etiology. Episodes are characterized by macroscopic and microscopic hematuria and mild proteinuria, often precipitated by upper respiratory tract infection. Laboratory values other than UA are normal. The diagnosis is confirmed by biopsy, which may not be needed if the disease is mild and confirmed in relatives. In the absence of notable proteinuria, deafness, ocular defects, renal failure, and with normal biopsy findings, the prognosis is excellent. Chronic nephritis is most commonly known as membranoproliferative GN and is distinguished by four types based on biopsy. Chronic nephritis can be found after acute nephritis or when investigating nonspecific complaints, 933such as anorexia,intermittent vomiting, and malaise. It is manifested by diminished renal function that ultimately has detrimental effects on other organ systems. Types I and II may respond to steroids, but the overall prognosis is guarded. Pyelonephritis, discussed earlier in the Pyelonephritis section, is inflammation of the renal parenchyma, calyces, and pelvis caused by bacteria. The inflammatory response of the kidneys results from various causes, such as infection, an immunologic response, a drug or toxin, and vascular or systemic disorders. PSGN is an immune response by the host to a group A beta-hemolytic streptococcal infection, whereas acute postinfectious glomerulonephritis (APGN) can be caused by bacterial, fungal, viral, parasitic, or rickettsial agents. PSGN is the most common form of nephritis in childhood, occurs most often between 5 and 12 years old, occurs more often in males (2 : 1), and is unusual in children younger than 3 years old. The incidence of APGN is difficult to determine because of the large number of patients with subclinical cases (Pan and Avner, 2011a). Clinical Findings History • Streptococcal skin (more likely) or pharyngeal infection within the past 2 to 3 weeks (PSGN). Classically a latent period of 7 to 10 days elapses between infection and the onset of symptoms; if fewer than 5 days or more than 14 days, consider other causes. • Abrupt onset of gross hematuria. • Reduced urine output (with diuresis in 5 to 7 days). • Lethargy, anorexia, nausea, vomiting, abdominal pain. • Chills, fever, backache (pyelonephritis). • Medication taken in the past few weeks. Physical Examination • Hypertension that is transient and resolves in 1 to 2 weeks • Edema, especially periorbital edema, or abrupt onset with weight gain • Circulatory congestion—dyspnea, cough, pallor, pulmonary edema if severe • Ear malformations • Flank or abdominal pain or a mass (in polycystic kidney or malignancy [e.g., Wilms tumor]) • Costovertebral angle tenderness (in pyelonephritis) • Rashes or arthralgias (with SLE, HSP, or impetigo) • Evidence of trauma or abuse Diagnostic Studies • UA with microscopic examination—tea color; elevated specific gravity; macrohematuria and microhematuria; proteinuria not exceeding the amount of hematuria; pyuria in PSGN; granular, hyaline, WBC, or RBC casts; and dysmorphic RBCs • Serum C3 or C4 (low early in disease, returning to normal in 6 to 8 weeks), total protein and albumin (elevated) • CBC, ESR, ASO titer (elevated), streptozyme test (positive), anti–deoxyribonucleic acid (DNA) antibody titer • Electrolytes, BUN, creatinine, and cholesterol • Fluorescent antinuclear antibody (SLE), hepatitis titers, sickle cell or hemoglobin electrophoresis, tuberculin PPD, and fluorescent treponemal antibody absorption (syphilis) Differential Diagnosis Acute nephritis also occurs as part of systemic illnesses, such as SLE, HSP, hemolytic-uremic syndrome, vasculitis, or as a reaction to drugs or irradiation. Management Consultation with a nephrologist is recommended in all cases PSGN treatment is supportive because resolution occurs spontaneously 95% of the time. The course does not seem to be affected by corticosteroids, immunosuppression, or other treatment modalities. During the peak of oliguria and hypertension in the first few days of illness, hospitalization may be required with fluid and sodium limitation and diuretic, antihypertensive, and antibiotic treatment if cultures are positive. Resolution occurs once diuresis begins. Gross hematuria persists for 1 to 2 weeks, urine can be abnormal for 6 to 12 weeks, and microscopic hematuria can persist for up to 2 years. Complement levels return to normal in 6 to 8 weeks (Pan and Avner, 2011a). • Acute nephritis—possible hospitalization with treatment, as described previously. • IgA nephropathy—annual follow-up with BP, UA, and determination of renal function.• Benign familial or hereditary nephritis—perform audiometry and review family medical history. Hereditary markers are being developed for this disease. • Benign recurrent nephritis—monitor UA and renal function every 1 to 2 years. • Chronic nephritis—a team approach is required to adequately provide care. Complications Prolonged oliguria and renal failure can occur if acute nephritis progresses. Hypertensive encephalopathy or congestive heart failure can occur secondary to PSGN. Irreversible parenchymal damage causes hypertension and renal insufficiency. Patient and Family Education, Prevention, and Prognosis Patients with PSGN may have macrohematuria or microhematuria for up to 6 to 12 months, but the long-range outcome is excellent. Thinbasement-membrane disease has a good outcome. IgA nephropathy with severe histologic findings has a poor outcome, especially if the child is African American. Patient education should stress the importance of continued, regular care to monitor renal function. Osgood-Schlatter Disease Osgood-Schlatter disease is caused by microtrauma in the deep fibers of the patellar tendon at its insertion on the tibial tuberosity. The diagnosis is usually based on history and physical examination. The quadriceps femoris muscle inserts on a relatively small area of the tibial tuberosity. Naturally high tension exists at the insertion site. In children, additional stress is placed on the cartilaginous site as a result of vigorous physical activity, leading to traumatic changes at insertion. Osgood-Schlatter disease is often seen in the adolescent years after undergoing a rapid growth spurt the previous year. It occurs more frequently in boys than girls, with a male-to-female ratio of 3 : 1. This difference is probably related to a greater participation in specific risk activities by boys than by girls (Sullivani, 2015). Clinical Findings History. • Recent physical activity (such as, running track, playing soccer or football, or surfboarding) commonly produces the condition. • Pain increases during and immediately after the activity and decreases when the activity is stopped for a while. • Running, jumping, kneeling, squatting, and ascending/descending stairs exacerbate the pain. • The pain is bilateral in 20% to 50% of cases. • Approximately 25% of patients give a history of precipitating trauma. Physical Examination. Characteristic findings include the following (Sullivan, 2015): • Pain may be reproduced by extending the knee against resistance, stressing the quadriceps, or squatting with the knee in full flexion • Focal swelling, heat, and point tenderness at the tibial tuberosity • Full range of motion of knee Diagnostic Studies. The diagnosis is based on history and physical examination. Radiographs are not needed unless another pathologic condition is suspected. Differential Diagnosis Other knee derangements, tumors (osteosarcoma), and hip problems with referred pain should be considered. The referred pain of hip problems is diffuse across the distal femur without point tenderness at the tibial tubercle. Management Osgood-Schlatter disease is a self-limiting condition, with symptom management the key consideration. The following steps are taken: • Avoid or modify activities that cause pain until the inflammation subsides. • Ice or cold therapy to reduce pain and inflammation. • Once the acute symptoms have subsided, quadriceps-stretching exercises, including hip extension for complete stretch of the extensor mechanism, may be performed to reduce tension on the tibial tubercle. Stretching of the hamstrings may also be useful. • Use of NSAIDs is recommended by some but thought ineffective by others. Because this condition may last up to 2 years, their chronic use may be problematic. • A neoprene sleeve over the knee may help stabilize the patella. • A patella tendon strap that wraps around the joint just below the knee reduces the strain on the tibial tuberosity.• Cylinder casting or bracing with limited weight bearing for 2 to 3 weeks may be used in severe cases. Complications In the postpubertal child, a residual ossicle in the tendon next to the bone may cause persistent pain. Surgical removal is indicated and will relieve the pain. Prevention The condition cannot be prevented, but earlier management may decrease the length of disability and the discomfort 1069associated with it. Avoid overuse and encourage balanced training and adequate warm-up before exercise or sports participation. The use of kneepads may help protect the tibial tuberosity from direct injury for those who engage in sports that result in knee contact. Juvenile Idiopathic Arthritis JIA, formerly known as juvenile rheumatoid arthritis (JRA), now encompasses several disorders that have a common feature of arthritis (e.g., enthesitis-related arthritis and psoriatic arthritis) and had not been identified under the nomenclature of JRA (Wu et al, 2011). The diagnosis of JIA requires a persistent arthritis for more than 6 weeks in a pediatric patient younger than 16 years old. Table 25-1 shows the most current classification system. TABLE 25-1 Juvenile Idiopathic Arthritis Subtypes and Clinical Joint Characteristics Juvenile Idiopathic Arthritis Subtype Clinical Joint Characteristics Oligoarticular Four or less joints with persistent disease never having more than four-joint involvement and extended disease progressing to more than four joints within the first 6 months Polyarticular (RF negative) Five or more joints with symmetrical involvement Polyarticular (RF positive) Symmetric involvement of both small and large joints with erosive joint disease Systemic Either polyarticular or oligoarticular disease Enthesitis-related arthritis Weight-bearing joints involved especially hip and intertarsal joints and a history of back pain, which is inflammatory in nature or sacroiliac joint involvement Psoriatic arthritis Asymmetric or symmetric small or large joints UndifferentiatedRF, Rheumatoid factor. The underlying cause of most forms of JIA is unclear; however, it is a heterogenous disorder. It is likely environmentally induced in genetically predisposed individual. Human leukocytic antigen (HLA) class I and II alleles have been associated with JIA (Gowdie and Tse, 2012). This linkage points to the involvement of T cells and antigen presentation in the pathophysiology of the disease. An environmental trigger, such as infection or trauma, is also important in the pathogenetic process in JIA. The trigger results in an uncontrolled adaptive and innate response toward the self-antigen, the autoimmune reaction. The presence of autoantigens from cartilage and joint tissue leads to activation of the T cells and results in release of proinflammatory cytokines (Gowdie and Tse, 2012). In contrast, systemic juvenile idiopathic arthritis (SJIA), which does not have HLA gene association, may be the result of an autoinflammatory response from the innate immune system. SJIA is postulated to be the result of uncontrolled activity of the innate immune system, because this type of JIA disease is not associated with 552autoantibodies but rather uncontrolled activity of the phagocytes, including neutrophils, monocytes, and macrophages. The difference in the pathogenic processes may explain the differences in the clinical presentation of the disease. In oligoarticular and rheumatoid factor (RF)-positive polyarticular JIA, there is autoimmunity with involvement of the adaptive immune system. The presence of positive ANAs and RF is associated with HLA genes. The humoral response is responsible for the release of autoantibodies (especially ANAs), an increase in serum immunoglobulins, and the formation of circulating immune complexes and complement activation. The cell-mediated reaction is associated with a T-lymphocyte response that plays a key role in cytokine production, resulting in the release of tumor necrosis factor alpha (TNF-α), IL-1, and IL-6. B lymphocytes are activated by T-helper cells and produce autoantibodies that link to self-antigens. The B lymphocytes infiltrate the synovium with the end result of nonsuppurative chronic inflammation of the synovium that can lead to articular cartilage and joint structure erosion. The chronic arthritides of childhood present unique challenges to the child, family, and the pediatric provider. Approximately 1 in 1000 children are affected with oligoarticular JIA, the most common arthritic subtype. Certain histocompatibility complex antigens are more prevalent in the JIA population. Cytokine production, proliferation of macrophage-like synoviocytes, infiltration with neutrophils and T lymphocytes, and autoimmunity are thought to be the major pathologic processes causing chronic joint inflammation. The rate of JIA is significantly higher in girls than in boys, typically in oligoarticular and pauciarticular JIA. The female to male ratio in systemic onset is equal. The approximate percentage of occurrence and age breakdown for each of the subtypes follows: systemic (10%) occurs at any age; polyarticular (40%) has a late (6 to 12 years old) or early childhood (1 to 4 years old) onset; and oligoarticular (50%) has a late or early onset. Adolescents tend to have more RF-positive disease (Wu et al, 2011). Clinical Findings History The major complaints in all forms of JIA are from the arthritis characterized by: • Pain—generally a mild to moderate aching • Joint stiffness—worse in the morning and after rest; arthralgia may occur during the day • Joint effusion and warmth Systemic symptoms are found more commonly in systemic and polyarticular subtypes and include anemia, anorexia, fever, fatigue, lymphadenopathy, salmon-colored rash (SJIA), and weight loss. Growth abnormalities can result in localized growth disturbances, including premature fusion of the epiphyses, bony overgrowth (rheumatoid nodules), and limb-length discrepancies. Physical Examination Associated features are: • Non-migratory monoarticular or polyarticular involvement of large or proximal interphalangeal joints for more than 3 months • Systemic manifestations—fever, salmon-colored rashes, leukocytosis, serositis, lymphadenopathy, and rheumatoid nodules Less commonly seen are ocular disease (e.g., iridocyclitis, iritis, or uveitis), pleuritis, pericarditis, anemia of chronic disease, fatigue, and growth failure, or leg-length discrepancy if the arthritis is unilateral. Key physical findings are: • Swelling of the joint with effusion or thickening of synovial membrane, or both, noted on palpation of the joint line • Heat over inflamed joint and tenderness along joint line • Loss of joint range of motion and function; child typically holds the affected joints in slight flexion and may walk with limp • Uveitis may be present with ciliary injection and decreased vision. However, it is usually asymptomatic. There are five major types of JIA (Gowdie and Tse, 2012): 1. Oligoarticular pattern: This type of JIA involves four or less joints, typically the weight-bearing joints within the first 6 months of diagnosis. The diagnosis is classified as persistent or extended disease, depending on the number of joints involved. About 50% progress to extended disease where there is involvement of four or more joints after the first 6 months of disease. This involvement primarily is inlarger or medium joints, such as the knee, ankle, wrists, and elbow; however, systemic symptoms are rare. The synovitis may be mild and painless with asymmetric joint involvement and unremarkable laboratory values. Uveitis occurs in 30% especially if the child has a positive ANA (Gowdie and Tse, 2012). 2. Polyarticular pattern: This involves five or more joints and is divided into RF-negative and RF-positive disease. Involved joints can be large or small with an acute or insidious onset. RF-negative ANA positive polyarticular JIA is difficult to distinguish from extended oligoarticular pattern disease. Using the number of joints involved and the timing of onset of the arthritis can be helpful. In contrast, RFpositive disease can have chronic pain and symmetric joint swelling, low-grade fever, fatigue, nodules, and anemia of chronic disease. An acute form of uveitis occurs in this subtype. Polyarticular JIA typically involves small joints of the hands, feet, ankles, wrists, knees, and can also involve the cervical spine. Adolescents with this type differ from those with early onset in that they exhibit a positive RF. Adolescents who develop late-onset polyarticular JIA have a course similar to the adult entity. Both forms of the disease are more common in females. 3. SJIA: This is characterized by arthritis in one or more joints for 6 weeks' duration in a child younger than 16 years old with a fever of at least 2 weeks' duration with 553at least 3 days of daily fever. In addition, there is also a fleeting erythematous rash, lymphadenopathy, hepatomegaly, splenomegaly, and serositis (Ringold et al, 2013). Myocarditis with pericardial effusion occurs in approximately 10%. RF is rarely positive and the ANA is only positive in 5% to 10%; however, there may be anemia, thrombocytosis, increased acute phase reactants, and elevated transaminase levels. About 10% of children with SJIA develop a life-threatening macrophage activation syndrome (MAS) with fever, organomegaly, cytopenia, hyperferritinemia (acute phase reactant), hypertriglyceridemia, coagulopathy, and hypofibrinogenemia. 4. Enthesitis-related JIA: This typically entails arthritis of the lower limbs especially the hip and intertarsal joints with the sacroiliac joints involved later in the disease. Enthesitis involves inflammation at the insertion of tendons, ligaments, or joint capsules and is characterized by swelling, tenderness, and warmth. Enthesitis may present with joint or foot pain. There is a risk of anklyosing spondylitis 10 to 15 years later. It tends to occur in late childhood and adolescence and acute symptomatic uveitis occurs in about 7%. 5. Psoriatic arthritis: This is more common between the ages of 2 and 4 and again between 9 to 11 years old. There is usually a family history of psoriasis, or the child has psoriasis; however, the arthritis can precede the psoriasis by years. There can be dactylitis or a sausage-like swelling of the digits; involvement in the small digits is not uncommon. Diagnostic Studies JIA is a diagnosis of exclusion. The diagnosis is based on physical findings and history of arthritis lasting for 6 weeks or longer. There is no diagnostic laboratory test for JIA. Most children with oligoarticular arthritis have negative laboratory markers. Those with polyarticular and systemic-onset typically have elevated acute-phase reactants and anemia of chronic disease. A positive result for RF by latex fixation may be present, but a positive RF occurs in less than 10% of children with JIA and rarely in those with SJIA. ANA may be present in up to 50% of children with oligoarticular disease. A positive ANA helps identify children at higher risk for uveitis. The anti-CCP antibody test can be added to the initial workup of JIA, because citrullinated residues are part of the essential antigenic components that are recognized by autoantibodies in rheumatoid arthritis (Mehta, 2012). The anti-CCP antibodies are associated with more aggressive disease and may be present before the onset of symptoms. The anti-CCP antibody is highly specific, but its precise role has not been established because it is found primarily in children with polyarticular JIA (Mehta, 2012). Useful laboratory tests include a complete blood count (CBC) (to exclude leukemia); ESR, CRP, Lyme titers, and liver function tests. The results may reveal lymphopenia, anemia, elevated transaminases, and hypoalbuminemia; however, laboratory studies may be normal in these children. Imaging studies (MRI) can help in managing joint pathologic conditions. Analysis of synovial fluid is not helpful in the diagnosis of JIA. Differential Diagnosis The various causes of monoarticular arthritis are part of the differential diagnosis. However, Lyme disease must be excluded and other differentials, including tumors, leukemia, cancer, bacterial infections, toxic synovitis, rheumatic fever, SLE, spondyloarthropathies, inflammatory bowel disease, septic arthritis, and chondromalacia patellae, need to be carefully considered. Management A specialist in pediatric rheumatology should follow children with severe involvement. Ophthalmology referral and evaluation is critical in a child with a positive ANA. Uveitis needs immediate ophthalmologic management. It is most common in oligoarticular JIA and is highly associated with a positive ANA. Other pediatric subspecialists, such as orthopedists, pain management specialists, and cardiologists, may be consulted as needed. Therapy depends on the degree of local or systemic involvement. The main treatment goals are to suppress inflammation, preserve and maximize joint function, prevent joint deformities, and prevent blindness. Drug therapy is used to control the inflammation responsible for tissue injury with the goal of preventing permanent tissue changes, which is not always possible. Aggressive early treatment to induce a remission is a key consideration in JIA management in order to prevent deformity and improve outcomes and is now the goal of the practice guidelines for both polyarticular JIA and SJIA (Ringold et al, 2013, 2014). Aspirin therapy has largely been replaced with the use of nonsteroidal anti-inflammatory drugs (NSAIDs). Pharmacologic agents commonly used in the management of JIA include the following (Gowdie and Tse, 2012): • NSAIDs: Children with oligoarthritis generally respond well to NSAIDs (Taketomo et al, 2014). • Ibuprofen: 30 to 40 mg/kg/day three to four divided doses (maximum single dose is 800 mg; maximum daily dose 2400 mg/day) • Tolmetin: 20 to 30 mg/kg/day divided in three to four doses (maximum dose is 1800 mg/day) • Naproxen: 10 mg/kg/day in two divided doses (maximum dose is 1000 mg/day) • Indomethacin: Older than 2 years old, 1 to 2 mg/kg/day divided in two to four doses (maximum dose is 4 mg/kg/day); adults, 25 to 50 mg/dose two or three times/day (maximum dose is 200 mg/day) • Celecoxib: Older than 2 years old and adolescents (≥10 kg to ≤25 kg), 50 mg twice daily; >25 kg, 100 mg twice daily• Oral, parenteral, intraarticular corticosteroids: • Systemic arthritis: Can be used for 2 weeks as initial therapy for SJIA with involvement of more than four joints and a physician global assessment (using the Provider global assessment tool of disease activity) of 554less than 5 or a Provider global score of more than 5 without care about active joint involvement. Corticosteroids can be used as bridging therapy until other medications take effect (Ringold et al, 2013) • All the other types of arthritis: Prednisone in the lowest possible dose with optional intraarticular steroid injection (Ringold et al, 2014) • Disease-modifying antirheumatic drugs (DMARDs): Recent published guidelines vary related to the initiation of these agents depending on type of arthritis, joint involvement, and MD global assessment of functioning • Nonbiologic DMARD treatment: methotrexate, sulfasalazine, leflunomide (managed by pediatric rheumatologist) • Biologic DMARD treatment (managed by pediatric rheumatologist) • Short-acting agents: Anti-IL-1 anakinra is the first-line agent for SJIA with significant joint involvement and poor global functioning (Sterba and Sterba, 2013). • Long-acting agents: Rilonacept, canakinumab, and tocilizumab have long-acting activity (Sterba and Sterba, 2013). Rilonacept is a recombinant fusion protein with high affinity for IL-1β, IL-1α, and IL-1 receptors and a half-life of 8.6 days. Canakinumab is a humanized monoclonal antibody effective against IL-β with a half-life of 28 days. Tocilizumab is effective against IL-6 (Sterba and Sterba, 2013). • TNF-α agents: For example, etanercept (Enbrel, infliximab (Remicade), and adalimumab (Humira) soak up tumor necrosis factor, an immune-system protein, and block the inflammatory cascade. Methotrexate or anakinra is used in severe forms of JIA. • Intraarticular corticosteroid injections are used if there is severe joint involvement. • Pharmacologic therapy for uveitis is given as indicated by an ophthalmologist. Females with ANA-positive oligoarticular JIA are at high risk for uveitis and require slit-lamp examination every 3 to 4 months. The uveitis often does not correspond to the severity of the arthritis (i.e., uveitis may be present despite quiescent arthritis). • Physical therapy—range of motion muscle-strengthening exercises and heat treatments—is used for joint involvement; occupational therapy is beneficial. Rest and splinting are used if indicated. • Ophthalmologic follow-up every 3 months for 4 years (even if the arthritis has resolved) for all ANA-positive JIA children. They have a greater risk of uveitis that may not be clinically apparent but can lead to blindness if not detected and treated. Complications and Prognosis Systemic involvement can include iridocyclitis, uveitis, pleuritis, pericarditis, anemia, fatigue, and hepatitis. Residual joint damage caused by granulation of tissue in the joint space can occur. Children most likely to develop permanent crippling disability include those with hip involvement, unremitting synovitis, or positive-RF test. The course of the disease is variable, and there is no curative treatment. Again, early aggressive treatment is critical; therefore, referral to a specialist is important. After an initial episode, the child may never have another episode, or the disease may go into remission and recur months or years later. The disease process of JIA wanes with age and completely subsides in 85% of children; however, systemic onset, a positive RF, poor response to therapy, and the radiologic evidence of erosion are associated with a poor prognosis. Onset of disease in the teenage years is related to progression to adult rheumatoid disease. Patient and Parent Education and Prevention The following education and preventive measures are taken: • For children on aspirin therapy (not typically given), educate parents about the risk of Reye syndrome and its signs and symptoms. • Recommend yearly influenza vaccine. • Offer chronic disease counseling and encourage normal play and recreation. • Educate about side effects of medications, in addition to splinting, orthotics, and bracing requirements. • Instruct about need to follow up with an ophthalmologist. Frequency of follow-up for uveitis screening is based on subtype of JIA and is determined by protocol guidelines and ophthalmology. • Ensure parent and child understand that physical therapy is a mainstay of treatment for chronic childhood arthritis and should be part of the child's daily routine. A daily plan should include passive, active, and resistive exercises. • Water therapy and the use of heat or cold reduce pain and stiffness. Swimming is an excellent activity except for children with severe anemia and severe cardiac disease. • Tricycle or bike riding and low-impact dance are other beneficial activities. • Refer to the American Arthritis Foundation and the Juvenile Arthritis Association, which have excellent resources for family members and children. • Instruct on the need to involve school personnel in the identification of required school-related services through an individualized education plan (IEP) or a 504. • Discuss the challenge of pain management and its assessment in children with chronic arthritis and encourage parents to advocate for effective pain control on behalf of their child.Transient synovitis 3 to 8 years old + Mild to moderate fever, mild irritability; resolves within 1 week Limited hip motion; ESR <25 mm/h Inflammatory reaction; unknown etiology; often URI (50%) prior Rest Limps Deviations from normal age-appropriate gait pattern can be caused by a wide variety of conditions. A limp is usually mild and self-limited and caused by contusion, strain, or sprain. In some cases, the cause can be a sign of a serious inflammatory or infectious process. Age is an important factor in diagnosing the many causes of limping. Table 38-5 describes the various types of limps commonly seen in children. Clinical Findings History A careful history is needed, including: • Presence of pain • History of trauma, past medical history • Presence of fever, night sweats • Weight loss or anorexia • Type of limp (Table 38-6) • Interference with activities • Review of systems Physical Examination • Child should be unclothed during examination. • Observe for areas of erythema, swelling, atrophy, and deformity. • Observe each limb segment. • Identify limp type: Have the child walk and run while distracted. • Stance and swing phase should be compared in both legs. • Range of motion of each joint should be evaluated, especially the hip. • Complete a neurologic examination, including strength, reflexes, balance, and coordination. • Assess Trendelenburg sign for hip stability. Diagnostic Studies A CBC with differential and measurement of ESR and CRP levels should be obtained to rule out infection, inflammatory arthritis, or malignancy. Imaging should include radiographs of the area of concern. When imaging the hip, frog-leg lateral views should be obtained. Ultrasound may be used to detect effusion of the hip joint. If radiographs and ultrasound are positive, a CT scan may be indicated. Differential Diagnosis Fracture, DDH, LCPD, SCFE, tumor, infection, juvenile arthritis, and others should be considered (see Table 38-6). Management Refer the patient to an orthopedist immediately, unless the etiology is a mild strain or a local lesion that can be managed conservatively by the primary care provider. Legg-Calvé-Perthes Disease LCPD is a childhood hip disorder that results in infarction of the bony epiphysis of the femoral head. It presents as avascular necrosis of the femoral head. The basic underlying cause of LCPD is insufficient blood supply to the femoral head. There is an initial ischemic episode of unknown etiology that interrupts vascular circulation to the capital femoral epiphysis. The articular cartilage hypertrophies, and the epiphyseal marrow becomes necrotic. The area revascularizes, and the necrotic bone is replaced by new bone. This process can take 18 to 24 months. There is a critical point in these dual processes when the subchondral area becomes weak enough that fracture of the epiphysis occurs. At this time, the child becomes symptomatic. With fracturing, further reabsorption and replacement by fibrous bone occurs, and the shape of the femoral head is altered. Articulation of the head in the hip joint is interrupted. The bone re-ossifies with or without treatment; but without treatment, the femoral head flattens and enlarges, causing joint deformity. Lateral subluxation of the femoral head is associated with poor outcomes.Etiology is unclear, but certain risk factors have been identified in children. These include gender, socioeconomic group, and the presence of an inguinal hernia and genitourinary tract anomalies. Boys are affected three to five times more often than girls; incidence increases in lower socioeconomic groups and in children with low birth weights. The disease is bilateral in 10% to 20% of children. It affects children 4 to 8 years old. Clinical Findings History. There can be an acute or chronic onset with or without a history of trauma to the hip, such as jumping from a high place. • The most common presenting sign is an intermittent limp (abductor lurch), especially after exertion, with mild or intermittent pain. • The most frequent complaint is persistent pain in the groin, anterior hip region, or laterally around the greater trochanter. • Pain may be referred to the medial aspect of the ipsilateral knee or to the anterior thigh. • Some children may report limited range of motion of the affected extremity. Physical Examination. Findings may include the following: • Antalgic gait with limited hip movement • Trendelenburg gait resulting from pain in the gluteus medius muscle • Muscle spasm • Atrophy of gluteus, quadriceps, and hamstring muscles • Decreased abduction, internal rotation, and extension of the hip • Adduction flexion contracture • Pain on rolling the leg internally Diagnostic Studies. Routine AP pelvis and frog-leg lateral views are used to confirm the diagnosis, stage the disease, and follow disease progression and response to treatment. Radiographic findings can include smaller epiphysis, increased epiphyseal density, subchondral fracture line, lateralization of the femoral head, and other features. Changes in the epiphysis margin are discerned by the orthopedist and radiologist (Fig. 38-10). However, there may be no radiographic findings early in LCPD. Ultrasonography is useful in the preliminary diagnosis; capsular distention can be seen on sonographic images. Bone scans and MRI allow for precise localization of the bone involvement, but changes seen as bone marrow edema and joint effusions are nonspecific. CT is not typically used on a routine basis to evaluate patients with LCPD (Kim and Herring, 2014). Differential Diagnosis Acute and chronic infections, sickle cell disease, toxic synovitis, Gaucher disease, slipped capital femoral epiphysis (SCFE), osteomyelitis, juvenile rheumatoid arthritis, hemophilia, and neoplasm are included in the differential diagnosis. Management • Referral to an orthopedist is necessary. Because age of onset and the severity of LCPD can vary significantly from one child to another, there are various approaches to the management, and treatment remains controversial. Overall, the general approach is guided by the principle of containment of the femoral head within the acetabulum. To be successful, containment must be instituted while the femoral head is still moldable. Non-operative containment can be achieved in a variety of ways and ranges from activity limitation, and protected weight-bearing, use of NSAID and physical therapy to maintain hip motion to bed rest with traction using casts to maintain hip abduction. Surgical approaches involve pelvic and femoral osteotomies of the proximal femur or pelvis. • Support and monitor the child throughout treatment and recovery, including during interruption of school or other activities. Treatment and monitoring of LCPD can last months to years. Complications Osteoarthritis related to femoral head deformity and decreased use of the hip joint may occur, depending on the femoral head remodeling status. Older children have a poorer prognosis owing to the decreased opportunity for femoral head remodeling in the remaining growth period. Females with LCPD also have a poorer prognosis. Prevention The condition is not preventable, but early identification and treatment reduce the long-term complications of the disorder, such as premature degenerative arthritis in early adult life.Idiopathic Scoliosis Adolescent scoliosis can resolve, remain static, or increase. As a result treatment options vary considerably. Treatment decisions are based on the natural history of each curvature. Infantile scoliosis can resolve spontaneously; however, progressive curves require bracing and surgery in an attempt to slow the curve progression and prevent complications (e.g., thoracic insufficiency syndrome). Juvenile scoliosis is found more frequently in girls, and the curves are at high risk for progression and often require surgical intervention. The goal in treatment is to delay spinal fusion, allowing time for the pulmonary system and thoracic cage to have matured and maximum trunk height to be achieved. (See the various surgical procedures described in the following section.) The natural history includes the degree of skeletal maturity or growth remaining, the magnitude of the curve, and any associated diagnoses or medical conditions. Observation is always indicated for curves less than 20 degrees. Bracing or surgery may be indicated for larger curves. Brace treatment may reduce the need for surgery, restore the sagittal profile, and change vertebral rotation. Indications for bracing are a curve more than 30 degrees. Additional indications for brace therapy include skeletally immature patients with curves of 20 to 25 degrees that have shown more than 5 degrees of progression. The efficacy of bracing for adolescent idiopathic scoliosis remains controversial. Some studies show brace treatment to be effective in preventing curvature progression; however, it has been found that the success of the treatment is proportional to the amount of time that the patient wears the brace. Various brace treatment protocols suggest wearing a brace as much as 23 hours per day; therefore, compliance is a significant factor for this treatment modality (Spiegel and Dormans, 2011). Surgical treatment is indicated for children and adolescents who have progressive spinal deformity that do not respond to bracing and for those with curvature exceeding 45 to 50 degrees (Richards et al, 2014). There are various surgical procedures; all aim to control progressive curvatures. In the past, surgery was limited to arthrodesis (surgical fusion) of the spine. In recent years, several procedures have been developed that are designed to postpone and, in some cases, eliminate the need for early spinal fusion and allow for growth. These include the vertical expandable prosthetic titanium rib (VEPTR). This procedure is indicated for children with restricted pulmonary function due to the curvature of their thoracic spine. The surgery involves implanting a prosthesis that serves to enlarge the constricted thorax. The prosthesis can be adjusted approximately every 4 to 6 months, thereby allowing for growth. The “growing rod” surgical procedure has shown success in patients with adolescent idiopathic scoliosis and involves inserting spinal rods that are used to exert distraction forces that are adjusted approximately every 6 months. The rods serve as an internal brace to control the curvature of the spine while allowing skeletal growth. A more recent procedure involves intervertebral spinal stapling or tethering. Unlike the VEPTR and growing rod procedures, intervertebral spinal stapling does not require repeat adjustments and, therefore, eliminates the need for repeat surgical procedures. Research on this technique is limited, and clinical indications have not been universally agreed upon. Further research is necessary and long-term results are yet to be determined. Referral to an orthopedist or a center that specializes in working with infants and children with scoliosis is essential. Support must be given to the child and family 1060through the diagnostic and treatment phases, considering school and peer factors. The primary care provider needs to assist the child with psychological adjustment issues that arise if bracing or surgery is recommended and instituted. Some specific concerns of the child can include self-esteem problems, managing hostility and anger, learning about the disease and its care, wondering about the long-term prognosis, and concerns about clothing and participation in sports and other activities. Complications Progressive scoliosis can result in a severe deformity of the spinal column. Severe deformities can result in impairment of respiratory and cardiovascular function and limitation of physical activities and decreased comfort. The psychological consequences of an untreated scoliosis deformity can be severe. Prevention Prevention is not possible; however, screening and early identification of children with scoliosis may help avoid more expensive, invasive care and prevent the long-term consequences of the disorder. Screening is effective, however, only if identified children are referred for care. Parents must be notified, a referral arranged, and follow-up ensured. Puncture Wounds Description and Epidemiology Puncture wounds result from penetration of varying levels of skin and underlying tissue. These wounds are typically classified as superficial or deep. Glass, wood splinters, toothpicks, needles, nails, metal, staples, thumbtacks, and bites are common sources of injury. Although the majority of puncture wounds heal without problems, a sizable minority of these injuries are complicated by infections that can lead to cellulitis, fasciitis, septic arthritis, or soft-tissue abscesses. Staphylococcus aureus and beta-hemolytic streptococci are normal flora of the skin and are common causesof secondary infections in puncture wounds. Pseudomonas aeruginosa colonizes on the rubber soles of tennis shoes and is a common pathogen for plantar puncture wounds when the puncture occurs through the sole of a tennis shoe and into the foot. Osteomyelitis can occur if the puncture wound penetrates a bone or joint and is most commonly caused by P. aeruginosa in nondiabetic patients and is most commonly caused by S. aureus in diabetic patients (Baddour, 2013). Cat and dog bites can cause wound infection from Pasteurella multocida. When considering risk for infection, the location and depth of the wound and the presence of a foreign object are important components. For example, deep penetrating injuries to the forefoot with a dirty object, especially if they involve the plantar fascia, have a higher risk of infection than wounds to the arch or heel area. The forefoot has less overlying soft tissue than other plantar surfaces and is the major weight-bearing area of the foot; therefore, cartilage and bone can be involved. The metatarsophalangeal joint region is also at high risk for infection for the same reasons. Puncture wounds through the soles of tennis shoes can transfer bacteria into the tissue while simultaneously impairing wound drainage, placing the child at higher risk for a secondary infection. Assessment The assessment of a child with a minor wound begins by excluding more serious and sometimes occult injuries. History. Important information to elicit after a report or suspicion of a puncture wound includes the following: • Date and time of injury and history of wound care provided at time of injury and thereafter. • Identification of the penetrating object and the type and estimated depth of penetration. If it is not known what object penetrated the skin, the likelihood of an imbedded foreign body is high. • Location and condition of the penetrating object. Was the object clean or rusty, jagged or smooth? • Whether all or part of the foreign object was removed. • Type and condition of footwear that was being worn (pertinent to injuries to the foot) or if the child was barefoot. • Immunization status for tetanus coverage (see Chapter 24). • Presence of any medical condition that increases the risk for infectious complications. Physical Examination. A good light source is necessary to assess and treat a puncture wound. Note circulation, movement, and sensation of the area next to the injury. Determine the amount of involvement of underlying tissue or bone structures. For plantar puncture wounds, have the patient lie prone with the feet positioned at the head of the examining table and the knees slightly flexed (Buttaravoli and Leffler, 2012). Assess the wound for length and depth, presence of debris or penetrating object, and signs of infection. Examination findings consistent with cellulitis include: • Localized pain or tenderness, swelling, and erythema at the puncture site (may be more obvious at dorsum of the foot for plantar puncture wounds) • Possible fever • Pain with flexion or extension of the extremity involved • Decreased ability to bear weight • For plantar puncture wounds, pain along the plantar aspect of the foot during extension or flexion of the toes may indicate deep tissue injury, thus a higher risk of infection Examination findings consistent with osteomyelitis-osteochondritis include: • Extension of pain and swelling around the puncture wound and to the adjacent bony structures • Exquisite point tenderness over the bone • Fever • Increasing erythema • Decreased use of the affected extremity Examination findings consistent with pyarthrosis (septic arthritis) include: • Pain, swelling, warmth, and erythema over the affected joint • Decreased range of motion and weight bearing of the affected joint • Fever Diagnostic Studies. Plain film radiograph should be ordered if any of the following occur:• A suspicion of a retained foreign object. • There is a tremendous amount of pain at the site of the wound, localized tenderness is noted over the wound, there is discoloration underneath the skin surface, or there is a palpable mass noted at or near the wound entry site (Baddour, 2013). • There was penetration of a joint space, bone or growth cartilage, or the plantar fascia of the foot. • The puncture site has signs of infection and is from a nail injury. • Most metal and glass foreign bodies can be seen on a plain radiograph. However, if the foreign object is not radiopaque or if the x-ray is negative despite suspicion of foreign object in the wound, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) are useful diagnostic tools (Buttaravoli and Leffler, 2012). • Bone scans are sensitive but not specific for osteomyelitis. Radiographs are specific, but findings for osteomyelitis are noted late. Clinical examination and laboratory studies and imaging should be considered early in the diagnosis of osteomyelitis (Erickson and Caprio, 2014). • A complete blood count (CBC) and blood culture may be needed. An elevation in the white blood cell count might indicate infection. • An erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are nonspecific inflammatory markers and are helpful in the diagnosis and management of bony inflammation and infection. • A wound culture is indicated prior to starting antibiotics if the wound appears infected. Differential Diagnosis The history and physical examination provide the diagnosis. Management The circumstance surrounding the penetrating injury and the presenting symptoms are the best indicators of whether the injury is superficial and will heal uneventfully or if it will result in infectious complications. Buttaravoli and Leffler (2012) suggest the following practical and straightforward approach to the management of puncture wounds: • For the majority of superficial or simple puncture wounds, débridement with an antiseptic solution after scrubbing the wound surface is sufficient. Consider the use of wound irrigation. Ensure that there are no foreign bodies present. • A No. 10 scalpel may be used to gently shave off the cornified epithelium surrounding the puncture wound to aid in the removal of debris that collected around the point of entry. • For wounds where debris is noted, gently slide the plastic sheath of an over-the-needle catheter down the wound track and move the catheter sheath in and out while irrigating with copious amounts of normal saline until debris no longer flows from the wound. A local anesthetic agent may be necessary for débridement and irrigation procedures. • Obtain imaging studies as indicated. If imaging studies demonstrate that the foreign object has invaded bone, growth cartilage, or a joint space, refer the child immediately to an orthopedic surgeon. Always suspect a retained foreign object if the puncture wound is infected, the infection is not responding to antibiotic therapy, or if pain or aching of the injured site is still present weeks after the injury. In order to prevent a catastrophic outcome, wounds that are deep or highly contaminated should be referred to an orthopedic surgeon so that débridement can take place in an operating room (Buttaravoli and Leffler, 2012). • Following careful wound cleansing, the wound can be covered with a simple bandage. Deeper wounds that require more extensive exploration should have a small sterile wick of iodoform gauze placed in the wound track in order to keep the edges open, thus aiding in granulation tissue growth and wound healing. Remove the gauze 2 to 3 days after placement (Selbst and Attia, 2010). • Children with simple, uncomplicated puncture wounds do not need antibiotics; however, if there are signs of infection, the puncture is the result of a cat bite, or if the wound is deep or contained debris, antibiotics should be part of the treatment plan. Appropriate antibiotics for puncture wounds include amoxicillin clavulanate or cephalexin. Clindamycin should be used when children are allergic to penicillins. Plantar puncture wounds require ciprofloxacin. If methicillin-resistant Staphylococcus aureus (MRSA) is cultured from the wound or pus is present at the puncture site, then trimethoprim-sulfamethoxazole (TMP-SMX) or clindamycin is recommended until sensitivities are known. All antibiotics should be prescribed for 7 to 14 days depending on severity of infection (Baddour, 2013). A recheck appointment should be scheduled 48 hours from the start of antibiotics for the patient receiving outpatient therapy.• Surgical débridement for removal of a foreign body and/or abscess drainage should be considered with an infected puncture wound (Baddour, 2013). • Treatment for severe infections secondary to puncture wounds, such as septic arthritis and osteomyelitis, includes surgical débridement and parenteral antibiotics (Hosalkar et al, 2011). • Tetanus prophylaxis is indicated if it has been more than 5 years since the last tetanus vaccine or if the date of the last tetanus vaccine is unknown. Consider passive immunization with tetanus immune globulin (TIG) or initiation/continuation of a primary tetanus series (DTaP, Tdap, or Td as appropriate) for children who have never been immunized or are behind in their vaccinations (see Chapter 24). Patient and Parent Education Home care management for a puncture wound includes: • Cleanse the wound two times a day and when soiling of the wound occurs. Use warm water and soap, and then apply bacitracin or triple antibiotic ointment to the wound. • Cover the wound with a dressing, such as an adhesive bandage. • Observe closely for signs and symptoms of infection and if infection is suspected, notify the provider immediately; rapid reevaluation is necessary. Further evaluation is required if a puncture wound continues to cause localized or spreading pain or discomfort. Croup (Laryngotracheitis and Spasmodic Croup) Croup is an acute, inflammatory disease of the larynx, trachea, and bronchi that clinically presents with a brassy cough that sounds like a bark and is associated with varying degrees of inspiratory stridor, hoarseness, and respiratory distress. Croup (laryngotracheitis and spasmodic croup) causes disease in children younger than 6 years old. The most common form of croup is typically termed laryngotracheitis. A viral infection of the glottic region extending into the subglottic region is called laryngotracheobronchitis (LTB), which is the term reserved for the more severe form of croup. It is an extension of laryngotracheitis occurring 5 to 7 days into the disease and is associated with bacterial superinfection (Roosevelt, 2011). Human parainfluenza types 1 and 2 (less so) are the most common viral agents responsible for fall outbreaks in children 1 to 6 years old, typically in odd numbered years. Other causative agents include other human parainfluenza types (notably HPIV- 3), influenza A and B, human coronavirus HL-63, coxsackieviruses, echoviruses, metapneumovirus, adenoviruses, RSV, and rhinovirus (Mejias and Ramilo, 2012). Viral croup is most common in children between 6 and 36 months old (60% are younger than 24 months), and it occurs most often in fall and winter. HPIV-3 is endemic in children younger than 6 months old and occurs in the spring and summer months, less commonly in the autumn if other parainfluenzae viruses are absent (Fox and Christenson, 2014). The incubation period is 2 to 4 days with viral shedding for up to 1 week before the onset of the disease (can shed up to 3 to 4 weeks with HPIV- 3) (Mejias and Ramilo, 2012). See Chapter 24 for more discussion about human parainfluenza viruses. Males are affected more often than females. Recurrent croup and recurrent laryngitis can develop in children until they are 6 years old. A positive family history has been noted in a small percentage of children in whom croup develops. Croup lasts approximately 5 days. With growth, the child's laryngeal tracheal airway is less vulnerable to the effects of viral infections and less susceptible to obstruction. Clinical Findings Clinical manifestations depend on the infectious agent responsible for the croup and the extent of the upper airway involvement. History The history typically includes the following: • URI prodromal symptoms (rhinorrhea, conjunctivitis, or both) are sometimes present before stridor • Acute onset of a hoarse, barking-like cough • Mild to severe laryngeal obstruction • Mild to severe inspiratory stridor with dyspnea • Gradual onset of symptoms (2 to 3 days) • Symptoms worse at night (Fox and Christenson, 2014) • May or may not have sore throat • Duration is generally 3 to 5 days for viral croup • The presence of fever without reoccurrence differentiates it from spasmodic croup (Fox and Christenson, 2014)Physical Examination The following can be seen: • Slight dyspnea, tachypnea, and retractions • Mild, brassy, or barking cough (harsh sounding) • Stridor—a high-pitched, harsh sound from turbulent airflow that is generally inspiratory, but may be biphasic • Temperature is typically low grade, but may be elevated to 104° F (40° C) • If visualized on examination of the mouth, the epiglottis will appear normal • Substernal and chest wall retraction in severe cases • Prolonged inspiration • Wheezing and rales may be heard if there is additional lower airway involvement Diagnostic Studies Croup is a clinical diagnosis. Radiography of the soft tissues of the neck and chest displays a classic pattern of subglottic narrowing (―steeple sign‖) on posteroanterior views but is usually not done unless there is a question about the diagnosis. Microbiology cultures of the pharynx can be helpful in selected cases that have atypical presentations with severe fever, toxic presentations, and severe inspiratory stridor because this presentation is more typical of bacterial tracheitis. 814 Differential Diagnosis Differential diagnoses include acute epiglottitis; acute spasmodic croup (no signs of infection); FB aspiration; retropharyngeal abscess; extrinsic compression from tumors, trauma, or congenital malformations; angioedema (anaphylaxis) or early asthmatic attack; bacterial tracheitis, infectious mononucleosis; and psychogenic stridor (Roosevelt, 2011). Croup is different from bacterial tracheitis, which is a bacterial infection of the trachea that occurs rarely and results in inflammatory cell infiltration of the larynx, trachea, and bronchi causing epithelial lining sloughing and mucopurulent membranes (Rajan, 2012). Bacterial tracheitis is a rapidly progressive disease of children 3 weeks to 16 years old accompanied by high fever. The most common ages are between 5 to 7 years old; it is associated with airway obstruction (Roosevelt, 2011). Staphylococcus is the most common organism, but M. catarrhalis, nontypable H. influenzae, and, less commonly, anaerobic organisms cause this disease (Roosevelt, 2011). Unlike viral croup, bacterial croup results in thick pus within the trachea and lower airways. These patients do not respond to the standard croup treatment and get clinically worse. Table 32-4 differentiates acute laryngotracheitis from other common causes of stridor. TABLE 32-4 Differentiating Common Respiratory Diseases That Can Cause Stridor or Similar Signs Radiographic view with findings Lateral or AP of neck/subglottic narrowing Lateral of neck/thumb sign Lateral of neck/subglottic narrowing Signs of obstruction in severe cases May see localized hyperinflation, mediastinal shift, atelectasis Treatment Humidification, corticosteroids in selected cases Hospitalization, cephalosporin, corticosteroids Hospitalization, staphylococcus coverage Hospitalization, erythromycin/penicillin, antitoxin FB removal, treatment of secondary infection or bronchospasm Intubation Rare Usually necessary Frequently necessary May be necessary Endoscopy to remove FB Prevention None Immunization—HIB None Immunization—DTaP Education on child-proofing home and monitoring childAP, Anteroposterior; CBC, complete blood count; DTaP, diphtheria-tetanus-acellular pertussis; FB, foreign body; HIB, Haemophilus influenzae type B; RSV, respiratory syncytial virus; URI,upper respiratory infection. Management Therapy depends on the cause, severity, and location of the disease. The aim of therapy is to provide adequate respiratory exchange. Table 32-5 shows management of the patient based on the degree of severity of croup. • Humidified air: A Cochrane review showed no evidence that inhalation of humidified air improved the outcome in croup scores in children with mild to moderate croup (Moore and Little, 2006). There is no evidence that the use of steam or cold humidification is harmful. However, cold air can be helpful. For a child with LTB, taking the child out into the cold night air or putting them by an opened freezer door may be beneficial. Often a ride in a car at night with the windows down accomplishes the same result. There is no evidence for the use of steam or humidification in croup. Occasionally, vomiting relieves the bronchospasm. • Nebulized epinephrine: Nebulized epinephrine has shown benefit in treatment of croup (Bjornson et al, 2013). • Corticosteroids: Corticosteroids decrease inflammation and cell damage without prolonging the viral shedding duration. In patients with croup, dexamethasone, oral or IM (0.6 mg/kg) or nebulized budesonide is beneficial, decreasing return visits and resulting in shorter hospital stay (Russell et al, 2011). IM dexamethasone can be used in a vomiting child. The rate of oral absorption equals that of IM therapy (Cherry, 2009a). The use of these agents should be limited to 1 to 2 days to avoid immunosuppression and the possibility of secondary bacterial infection. Dexamethasone has been shown to reduce inflammatory edema and to prevent destruction of ciliated epithelium (Cherry, 2009a). Antibiotics are not indicated. • Cold medications: Cough and cold medicines are not indicated in croup. • Bronchodilators: If bronchospasm is also suspected, the use of bronchodilators in the usual doses prescribed for relief of asthma (as discussed in Chapter 25) may be advantageous. • Oxygen: Blow-by oxygen is only used if the oxygen saturation falls below 92%. • Other modalities: In severe croup, a helium-oxygen mixture known as heliox can be used. TABLE 32-5 Croup Severity and Treatment Based on Severity Symptoms and Treatment Mild Moderate Severe Impending Respiratory Failure Symptoms Occasional croupy cough No retractions No chest wall retractions Frequent croupy cough Audible stridor and suprasternal and sternal retractions at rest No agitation Frequent croupy cough Tachypnea Prominent inspiratory and occasional expiratory stridor Agitation and distress Audible stridor at rest Sternal retractions Lethargy Decreased level of consciousness with dusky color Treatment Education of parent X X X X Corticosteroid X X X X Nebulized epinephrine X X Blow-by oxygen X X until intubationSymptoms and Treatment Mild Moderate Severe Impending Respiratory Failure Intubation X Data from Alberta Clinical Practice Guidelines Working Group: Guidelines for diagnosis and management of croup, Canada, 2003; Alberta, ON. Indications for Hospitalization Children in distress with respiratory rates between 70 and 90 breaths per minute or exhibiting stridor at rest should be hospitalized. A child with a temperature higher than 102.2° F (39° C) should be carefully evaluated; hospitalization may be necessary if other worrisome symptoms are present. Racemic epinephrine by aerosol may help but should be used in conjunction with corticosteroids to limit rebound swelling (dexamethasone 0.5 to 2 mg/kg/dose every 8 hours IV). Hydration is important (Fox and Christenson, 2014). IV fluids may be needed in patients who cannot tolerate feedings. Complications Increasing obstruction of the airways causes continuous stridor, nasal flaring, and suprasternal, infrasternal, and intercostal retractions. With further obstruction, air hunger and restlessness occur and are quickly followed by hypoxia, weakness, decreased air exchange, decreased stridor, increased pulse rate, and eventual death from hypoventilation. Anything that taxes the child's respiratory efforts, such as crying or feeding, causes more respiratory distress. Examination of the nasopharynx with a tongue depressor may result in sudden respiratory compromise. Severely ill children should be evaluated for acute epiglottitis or bacterial tracheitis. Viral pneumonia complicates about 1% to 2% of croup cases. Respiratory syncytial virus immune globulin RSV-IGIV (RespiGa m) Reduces risk of RSV bronchiolitis or pneumonia in high-risk children Provides additional protection against other respiratory viral illnesses; may be preferred over palivizumab in children with immune deficiencies or for premature infants prior to discharge in the RSV season for the first month of prophylaxis Palivizumab, a monoclonal antibody, is generally preferred over RSV-IGIV (see Chapter 32, Bronchiolitis) Asthma Asthma is a chronic respiratory disease characterized by periods of coughing, wheezing, respiratory distress, and bronchospasm. Asthma can occur with a persistent cough without significant wheezing. It is the most common chronic respiratory disease of children, with an incidence as high as 30% of children in the Western world, and it is the leading cause of emergency department visits (Jackson et al, 2011; Liu et al, 2011). The pathophysiology is the result of immunohistopathologic responses that produce shedding of airway epithelium and collagen deposition beneath the basement membrane, edema, mast cell activation and inflammatory infiltration by eosinophils, lymphocytes (Th2-like cells), and neutrophils (especially in fatal asthma). The persistent inflammation can result in irreversible changes, such as airway wall remodeling. Inflammation causes acute bronchoconstriction, airway edema, and mucous plug formation. In addition, airway inflammation can trigger a hyperresponsiveness to a variety of stimuli, including allergens, exercise, cold air, and physical, chemical, or pharmacologic agents. This results in bronchospasm, which presents as wheezing, breathlessness, chest tightness, and cough that can be worse at night or with exercise. The airflow obstruction is often reversible, either spontaneously or with treatment. Remodeling of the airway can occur secondary to persistent fibrotic changes in the airway lining. The fibrosis alters the airway caliber, leading to decreased airflow with permanent changes starting in childhood, but become recognizable in adults. Recent advances have shown that there are different ―phenotypes‖ of this disease with different clinical manifestations, and data suggest that children who have symptoms before 3 years old are more likely to have changes in lung functioning at 6 years old (Szefler et al, 2014). Asthma in children is classified as intermittent, mild persistent, moderate persistent, or severe persistentdepending on symptoms, recurrences, need for specific medications, and pulmonary function measurements (Table 25-2). Children classified at any level of asthma can have episodes involving mild, moderate, or severe exacerbations. Exacerbations involve progressive worsening of shortness of breath, cough, wheezing, chest tightness, or any combination of these symptoms. The degree of airway hyperresponsiveness is usually related to the severity of asthma that can change over time. A wellcontrolled child with asthma has only one exacerbation in 3 years on average (Jackson et al, 2011). Many children experience early- and late-phase responses to their asthma episode. The early asthmatic response (EAR) phase is characterized by activation of mast cells and their mediators, with bronchoconstriction being the key feature. EAR starts within 15 to 30 minutes of mast cell activation and resolves within approximately 1 hour if the individual is removed from the offending allergen. The latephase asthmatic response is a prolonged inflammatory state that usually follows the EAR within 4 to 12 hours after exposure to the allergen, is often associated with airway hyperresponsiveness more severe than the EAR presentation, and can last from hours to several weeks. Exercise-induced bronchospasm describes the phenomenon of airway narrowing during, or minutes after, the onset of vigorous activity. Most asthmatics exhibit airway hyperirritability after vigorous activity and display exercise-induced bronchospasm. For some children, exercise is the trigger for their asthma. Although asthma is not always associated with an allergic disorder in children, many pediatric patients with chronic asthma have an allergic component. Increased weight gain in pregnancy and the first 2 years of life may increase TNF-α, a proinflammatory cytokine implicated in asthma, which may be a predictive biomarker for asthma (Szefler et al, 2014). It is not known for certain whether hyperresponsiveness of the airways is present at birth or acquired later in genetically predisposed children. However, the genetic predisposition for the development of an IgE-mediated response to common aeroallergens, known as atopy, remains the strongest identifiable predisposing risk factor for asthma. A combination of genetic predisposition and exposure to certain environmental factors are the necessary components responsible for the pathophysiologic response associated with asthma. Origins of asthma exacerbations include exposure to respiratory virus, seasonal patterns, exposure to mycoplasma pneumonia and Chlamydophila pneumoniae, pollution, smoking, pregnancy, and psychological stress (Jackson et al, 2011; Szefler, 2013). Asthma is rarely diagnosed before 12 months old due to the high rate of viral illness causing bronchiolitis (Nelson and Zorc, 2013). A diagnosis of asthma should be made with caution in a toddler who has only wheezing associated with viral infections (Mueller et al, 2013). The morbidity and mortality statistics of asthma in childhood demonstrate an alarming increasing incidence of asthma and its complications with a lifetime prevalence of 13% (Nelson and Zorc, 2013). Theprevalence rate for asthma is highest among children 5 to 17 years with the highest rate among black children (Centers for Disease Control and Prevention, 2015). Minority children have fewer ambulatory care visits for asthma and are less likely to be on a controller medication. Occupational or environmental exposure can cause airway inflammation associated with asthma. Factors known to precipitate or aggravate asthma in children include the following: • Atopic individual response to allergens—inhaled, topical, ingested • Viral infections and bacterial infections with atypical mycobacterium • Exposure to known irritants (paint fumes, smoke, air pollutants) and occupational chemicals • Gastroesophageal reflux • Exposure to tobacco smoke (for infants, especially smoking by mother) • Environmental changes—rapid changes in barometric pressure, temperature, especially cold air • Exercise and psychological factors or emotional stresses (e.g., crying, laughter, anxiety attack, or panic or panic disorder) • AR and sinusitis • Drugs (e.g., acetaminophen, aspirin, beta-blockers) • Food additives (sulfites) • Endocrine factors (e.g., obesity) Allergen-induced asthma results in hyperresponsive airways. The majority of children with asthma show evidence of sensitization to any of the following inhalant allergens: • House dust mites, cockroaches, indoor molds • Saliva and dander of cats and dogs • Outdoor seasonal molds • Airborne pollens—trees, grasses, and weeds • Food allergy, including egg and tree nut Clinical Findings History In a primary care setting, asthma should be monitored using a standardized instrument, which may include the Asthma Control Test (ACT), Asthma Control Questionnaire, Asthma Therapy Assessment Questionnaire, Asthma Control Score, and other instruments as found in the guidelines summary (National Heart, Lung, and Blood Institute [NHLBI], 2007, p 17). The advantages of a standardized questionnaire are that it allows the health care provider to assess changes in the patient's asthma and alter the management plan as needed. However, data suggest the use of these tools is not effective in poorly controlled children in an acute setting (Szefler, 2014). The assessment of asthma symptoms allows providers to determine if the asthma is well controlled, less well controlled, or poorly controlled (Mueller et al, 2013). Well-controlled children have symptoms less than 2 days a week and use short-acting beta2-agonists (SABAs) less than twice a week, whereas less wellcontrolled patients have symptoms more than 2 days a week and likely need a step up in treatments. Poorly controlled children have symptoms during the day and may utilize SABAs several times a day. In primary care settings and the emergency department, the initial presentation is assessed based on the ability to talk in sentences, breathlessness, and alertness (Nelson and Zorc, 2013). Critical points to cover in the history of a child being seen for asthma include the following: • Family history of asthma or other related allergic disorders (e.g., eczema or AR) • Conditions associated with asthma (e.g., chronic sinusitis, nasal polyposis, gastroesophageal reflux, and chronic otitis media) • Complaints of chest tightness or dyspnea • Cough and wheezing particularly at night and in the early morning or shortness of breath with exercise or exertion (characteristic of asthma) • Seasonal, continuous, or episodic pattern of symptoms that may be associated with certain allergens or triggering agents • Episodes of recurrent ―bronchitis‖ or pneumonia • Precipitation of symptoms by known aggravating factors (upper respiratory infections, acetaminophen, aspirin) • Level of alertness Physical ExaminationTable 25-3 outlines the physical assessment findings correlated with asthma severity. Broadly speaking, the following may be seen on physical examination: • Heterophonous wheezing (different pitches but may be absent if severe obstruction) • Continuous and persistent coughing • Prolonged expiratory phase, high-pitched rhonchi especially at the bases • Diminished breath sounds • Signs of respiratory distress, including tachypnea, retractions, nasal flaring, use of accessory muscles, increasing restlessness, apprehension, agitation, drowsiness to coma • Tachycardia, hypertension or hypotension, pulsus paradoxus • Cyanosis of lips and nail beds if hypoxic • Possible associated findings include sinusitis, AD, and AR. TABLE 25-3 Physical Assessment of Asthma and Asthma Severity Severity of Asthma Physical Assessment Findings Mild Wheezing at the end of expiration or no wheezing,No or minimal intercostal retractions along posterior axillary line. Slight prolongation of expiratory phase. Normal aeration in all lung fields. Can talk in sentences Moderate Wheezing throughout expiration. Intercostal retractions. Prolonged expiratory phase. Decreased breath sounds at the base Severe Use of accessory muscles plus lower rib and suprasternal retractions; nasal flaring. Inspiratory and expiratory wheezing or no wheezing heard with poor air exchange. Suprasternal retractions with abdominal breathing. Decreased breath sounds throughout base Impending respiratory arrest Diminished breath sounds over entire lung filed. Tiring, inability to maintain respirations Severely prolonged expiration if breath sounds are heard. Drowsy, confused Diagnostic Studies Laboratory and radiographic tests should be individualized and based on symptoms, severity or chronology of the disease, response to therapy, and age. Tests to consider include the following: • Oxygen saturation by pulse oximetry to assess severity of acute exacerbation. This should be a routine part of every assessment of a child with asthma. Pulse oximetry measures the oxygen saturation (SaO2) of hemoglobin—the percentage of total hemoglobin that is oxygenated. • A CBC if secondary infection or anemia is suspected (also check for elevated numbers of eosinophils). • Routine chest radiographs are not indicated in most children with asthma. Results are typically normal or only show hyperinflation. Again imaging should be ordered judiciously with consideration of the longterm risk. However, chest radiographs can be useful in the following situations: selected cases of asthma or suspected asthma or if the child has persistent wheezing without a clinical explanation. Children with hypoxia, fever, suspected pneumonia, and/or localized rales requiring admission are candidates for imaging. Infants with wheezing during the winter who have clinical bronchiolitis do not need imaging (Nelson and Zorc, 2013). • If sinusitis is suspected as the trigger, no diagnostic radiographic testing is needed. • Allergy evaluation should be considered, keeping in mind that history and physical examination are key in this consideration. (Refer child to pediatric allergist.) • Sweat test should be considered based on history in every patient with asthma. • Pulmonary function tests: • Spirometry testing is the gold standard for diagnosing asthma and should be used on a regular basis to monitor, evaluate, and manage asthma. Exercise challenges using spirometry can also be done to evaluate the child with exercised-induced asthma. Children older than 5 years can typically perform spirometry. • To evaluate the accuracy of the spirometry, look for an initial sharp peak with an extension down to the baseline at the end of expiration that is reproducible at least two times. Compare the child's values with the predicted value for the child's age, height, sex, and race. • Look at the forced expiratory volume in 1 second (FEV1), which represents the amount of air exhaled in 1 second. The interpretation of percentage predicted is: • >75%: Normal • 60% to 75%: Mild obstruction • 50% to 59%: Moderate obstruction • <49%: Severe obstruction • The forced vital capacity (FVC) represents the amount of air expelled:• 80% to 120%: Normal • 70% to 79%: Mild reduction • 50% to 69%: Moderate reduction • <50%: Severe reduction • The FEV1/FVC represents the amount of air expelled in the first second over the total amount of air expelled and should be greater than 90% of the predicted value. Spirometry testing is done prior to a breathing treatment and 10 minutes after the treatment. If the child's FEV1 improves by 12%, the child likely has asthma because this illustrates hyperresponsiveness. • The forced expiratory flow (FEF) (FEF25 to FEF75) reflects the middle portion of the downward limb of the curve and is a good measure of smaller airway function. The interpretation of percentage predicted is: • >60%: Normal • 40% to 60%: Mild obstruction • 20% to 40%: Moderate obstruction • <10%: Severe obstruction • Doing spirometry during well-child checks and for sick visits gives the practitioner an excellent indication of the amount of inflammation and bronchospasm present in the airway (Kamakshya, 2012). Table 25-4 represents abnormal spirometry patterns. TABLE 25-4 Abnormal Spirometry Findings Obstructive Restrictive FVC Normal or ↓ ↓ FEV1 ↓ ↓ FEV1/FVC ↓ Normal or ↑ FEV1, Forced expiratory volume in 1 second; FVC, forced vital capacity. • Consider the use of more sophisticated pulmonary laboratory studies for the child with severe asthma. • Peak flow measurements: • If spirometry is not an option, peak expiratory flow (PEF) can be used. • PEF can be used in some children as young as 4 to 5 years old. The values are instrument specific, so the child's personal best value is the best guide to help detect possible changes in airway obstruction. The predicted range for height and age can be substituted if personal best rate is not available (Table 25-5). Interpretation of PEF reading is as follows if PEF is in the: • Green zone: More than 80% to 100% of personal best signals good control. • Yellow zone: Between 50% and 79% of personal best signals a caution. • Red zone: Between 0% and 50% of personal best signals major airflow obstruction. • Box 25-3 describes use of peak flowmeter and interpretation of results.• Exhaled nitric oxide (Dweik et al, 2011): • A biomarker for the children with asthma is exhaled nitric oxide testing, which measures a fraction of exhaled nitric oxide (FEno). • The test measures eosinophilic airway inflammation and helps to determine whether corticosteroids would be helpful in the management of the patient. It may support the diagnosis of asthma and can help determine compliance with corticosteroid therapy. • A FEno value of more than 35 ppb in children indicates eosinophilic inflammation and likely responsiveness to corticosteroids, whereas 25 to 35 ppb should be interpreted with caution. There is still controversy about this test, although guidelines have been published. Differential DiagnosisNumerous conditions can cause airway obstruction and be incorrectly confused with asthma, especially in young children and infants. Differential diagnoses include: • Acute bronchiolitis, laryngotracheobronchitis, bronchopneumonia, pneumothorax • Bronchial foreign body aspiration • Congenital malformations of the heart with CHF • Congenital pulmonary abnormalities or bronchopulmonary dysplasia • Genetic disorders, such as cystic fibrosis and alpha-1-antitrypin deficiency • Tracheal or foreign body compression (e.g., vascular aortic ring, enlarged lymph nodes or tumors) • Chronic lower respiratory tract infections caused by immunodeficiency disorders • Congenital malformation of the GI system with resultant recurrent aspirations • Vocal cord dysfunction • Gastroesophageal reflux disease • Exposure to toxic substance • Anaphylaxis Management Management strategies are based on whether the child has intermittent, mild persistent, moderate persistent, or severe persistent asthma (see Table 25-3). A stepwise approach is recommended. If control of symptoms is not maintained at a particular step of classification and management, the health care provider first should reevaluate for adherence and administration factors. If these factors do not appear to be responsible for the lack of symptom control, go to the next treatment step. Likewise, gradual step-downs in pharmacologic therapy may be considered when the child is well controlled for 3 months. Inhaled corticosteroids may be reduced about 25% to 50% every 3 months to the lowest possible dose needed to control the child's asthma (NHLBI, 2007; Szefler et al, 2014). Chronic Asthma Treatment of chronic asthma in children is based on general control measures and pharmacotherapy. Control measures can include the following: • Avoid exposure to known allergens or irritants. • Avoid use of acetaminophen in children at risk for asthma (Jackson et al, 2011; McBride, 2011). • Administer yearly influenza vaccine. • Control environment to eliminate or reduce offending allergen. • Consider allergen immunotherapy. Studies have pointed to reduction in health care cost and improved outcomes associated with allergy immunotherapy (Dretzke et al, 2013; Hankin et al, 2013). • Treat rhinitis, sinusitis, or gastroesophageal reflux. • Other pharmacologic agents that may need to be considered include: • Anticholinergics—to reduce vagal tone in the airways (may also decrease mucus gland secretion) • Cromolyn sodium—to inhibit mast cell release of histamine • Leukotriene modifiers—to disrupt the synthesis or function of leukotrienes • If needed, refer to pulmonology for omalizumab, a recombinant DNA-derived, humanized IgG monoclonal antibody that binds to human IgE on the surface of mast cells and basophils. This anti-IgE monoclonal antibody is used as a second-line treatment for children older than 12 who have moderate to severe allergy-related asthma and react to perennial allergens. It is used when symptoms are not controlled by inhaled corticosteroids. • Follow up with PCP after an exacerbation requiring emergency department care, and obtain a clear written asthma action plan. • Education regarding asthma basics, including triggers and prevention with environmental modification, as well as the different treatment modalities includes the techniques of administration and dispelling any myths regarding asthma medication. In terms of coping, the child and family need to be able to understand their emotions, worries, and uncertainty, as well as when to contact their PCP. Developing and understanding the asthma action plan is very important during a well-child visit (Archibald and Scott, 2014). The pharmacologic management of asthma in children is based on the severity of asthma and the child's age. The stepwise approach to treatment (Figs. 25-1 and 25-2) is based on severity of symptoms and the use of pharmacotherapy to control chronic symptoms, maintain normal activity, prevent recurrent exacerbations, and minimize adverse side effects and nearly ―normal‖ pulmonary function. Within anyclassification, a child may experience mild, moderate, or severe exacerbations. NHLBI guidelines for assessing asthma control and initiating and adjusting asthma therapy for the various pediatric age groups are found in Figures 25-3 and 25-4.Important considerations to note in the pharmacologic treatment of asthma include the following: • Control of asthma should be gained as quickly as possible by starting at the classification step most appropriate to the initial severity of the child's symptoms or at a higher level (e.g., a course of systemic corticosteroids or higher dose of inhaled corticosteroid). After control of symptoms, decrease treatment to the least amount of medication needed to maintain control. • Systemic corticosteroids may be needed at any time and stepped up if there is a major flare-up of symptoms. Control of inflammation is a key principle in the management of asthma. • The combination of inhaled corticosteroids with a long-acting beta2-agonist (LABA) can further control asthma (Szefler, 2013). • Children with intermittent asthma may have long periods in which they are symptom-free; they can also have life-threatening exacerbations, often provoked by respiratory infection. In these situations, a short course of systemic corticosteroids should be used. • Variations in asthma necessitate individualized treatment plans. • β2 agonists can be administered with metered dose inhaler (MDI) therapy via spacer for children withmild and moderate exacerbations of asthma, but for children with severe airway obstruction who may have decreased deposition of drug in the base of the lung, a nebulizer may be better (Nelson and Zorc, 2013). There is need for more research on the use of MDI therapy and nebulizer therapy in the pediatric population (Szefler et al, 2014). A spacer or holding chamber with an attached mask enhances the delivery of MDI medications to the lower airways of a child. Spacers eliminate the need to synchronize inhalation with activation of MDI. Older children can use a spacer without the mask. • Dry powder inhalers (DPIs) do not need spacers or shaking before use. Instruct children to rinse their mouth with water and spit after inhalation. DPIs should not be used in children younger than 4 years old. • Different inhaled corticosteroids are not equal in potency to each other on a per puff or microgram basis. Tables 25-6 and 25-7 compare daily low, medium, and high doses of various inhaled corticosteroids used for children. Combination inhaled corticosteroid and LABA can be used in children from 4 years old (Taketomo et al, 2014). For treatment of exercise-induced bronchospasm: • Warm up before exercise for 5 to 10 minutes. • Use either an inhaled SABA or a mast cell stabilizer (cromolyn) or both prior to exercise. Combination of both types of drugs is the more effective therapy. A LABA can be used in older children. • Use two puffs of a β2 agonist and/or cromolyn MDI 15 to 30 minutes before exercise. Tolerance may develop if a β2 agonist is used more than a few times a week; it should not be used as a controller monotherapy. Those who exercise regularly and develop symptoms of asthma should use controller medication, preferably an inhaled corticosteroid. • Using a scarf or mask around the mouth may decrease exercise-induced asthma (EIA) induced by cold. Table 25-8 identifies the usual dosages for long-term control medications (exclusive of inhaled corticosteroids) used to treat asthma in children. Quick-relief medications are listed in Table 25-9. Practice parameters are guides and should not replace individualized treatment based on clinical judgment and unique differences among children. Acute Exacerbations of Asthma The treatment of acute episodes of asthma is also based on classification of the severity of the episode. Acute episodes are classified as mild, moderate, and severe. Signs and symptoms are summarized in Table 25-10. Early recognition of warning signs and treatment should be stressed in both patient or parent education, or both. The initial pharmacologic treatment for acute asthma exacerbations is shown in Figure 25-5. It consists of inhaled SABAs (albuterol), two to six puffs every 20 minutes for three treatments by way of MDI with a spacer, or a single nebulizer treatment (0.15 mg/kg; minimum 1.25 to 2.5 mg of 0.5% solution of albuterol in 2 to 3 mL of normal saline). If the initial treatment results in a good response (PEF/FEV1 > 70% of the patient's best), the inhaled SABAs can be continued every 3 to 4 hours for 24 to 48 hours with a 3-day course of oral steroids at 1 to 2 mg/kg/day to a maximum of 60 mg per day. Reassessment is important to ensure an adequate response and to further assess asthma severity. An incomplete response (PEF or FEV1 between 40% and 69% of personal best or symptoms recur within 4 hours of therapy) is treated by continuing β2 agonists and adding an oral corticosteroid. The β2 agonist can be given by nebulizer or MDI with spacer. Parents should be taught to call their PCP for additional instructions. If there is marked distress (severe acute symptoms) or a poor response (PEF or FEV1 <40%) to treatment, the child should have the β2 agonist repeated immediately and should be taken to the emergency department. Emergency medical rescue (911) transportation should be used if the distress is severe and the child is agitated and unable to talk. If children experience acute asthma exacerbations more than once every 4 to 6 weeks, their treatment plan should be reevaluated. This chapter focuses on the outpatient management of patient with asthma. However, familiarity with other drug options used in more severe asthma is important. They include: • Magnesium sulfate IV is used in emergency settings to decrease the intracellular calcium concentration. It causes bronchodilation due to respiratory smooth muscle relaxation. • Ipratropium oral inhalation is an anticholinergic bronchodilator used to treat bronchospasms. Evidence of its long-term maintenance use to control bronchospasms is lacking (Taketomo et al, 2014) • Epinephrine given subcutaneously or intramuscularly is still an option in severe asthma where thedelivery of medication to smaller airways is limited due to bronchoconstriction. • Heliox is a mixture of oxygen and helium, which can improve drug delivery in obstructed airways because helium has a lower density and less airway resistance (Nelson and Zorc, 2013). Complications Complications from asthma can range from mild secondary respiratory infections to respiratory arrest. Unresponsiveness to pharmacologic agents can lead to status asthmaticus and ultimately to death. Chronic high-dose steroid use leads to growth retardation and other related side effects. Patient and Parent Education and Prevention The PCP needs to support self-care management through in-depth education as appropriate. Easy to understand education needs to be tailored to meet the child's individual needs, family needs, and cultural beliefs using a ―teach back‖ technique. Correct administration of inhaled medication should be demonstrated during initial training sessions and reevaluated in subsequent visits. Provide instruction on the following: • Basic understanding of what asthma is, what is good asthma control, and what is the child's current level of symptomatology • Environmental control of allergens or triggers, such as smoking and dust • Basic understanding of what different medications do and how to use them: Give clear, written instructions on how to administer, how much and when to give, monitoring side effects, and how long medication should be taken. A written plan is highly recommended based on either symptoms or peak expiratory flow rate (PEFR). • How to use inhalers, spacer devices, or aerosol equipment (Box 25-4) along with proper cleaning of aerosol equipment Identifying asthma symptoms that indicate a change in therapy is needed or necessitate immediate reevaluation; when and where to seek emergency care • Home PEF monitoring or symptom monitoring: What to do if symptoms worsen (what medications to add or increase; how frequently to use inhaled medication; specific indications about when to seek additional medical treatment for worsening of symptoms) • Development of a written action/treatment plan with the child or parent to cover these issues (Fig. 25-6 shows a sample form for home treatment plan.) • Need to have an adequate supply of all medications (including oral corticosteroids) at home and medications readily accessible to the child at school or other settings where the child frequents • Management of the child at school, camp, or other places away from home • Need for regular follow-up every 1 to 6 months and as needed with exacerbations The PCP should stress that asthma is a chronic disease that can be controlled—the goal of therapy is to maintain normal activity. The absence of symptoms does not mean the disease has disappeared, rather that it is well controlled. The child should wear a medical alert bracelet. Children and parents should be acquainted with local asthma education programs and activities, such as asthma camp. Written instructions and handouts should be provided for other significant individuals in the child's life, including caregivers and school personnel. Prognosis Asthma is a chronic disease that for most children can be successfully managed with proper pharmacologic therapy, allergen and environmental control, and patient education. Mild asthma is more likely to disappear with increasing age than is moderate or severe asthma. Rhinosinusitis Inflammation and edema of the mucous membranes lining the sinuses cause obstruction and set up an ideal situation for bacteria to invade the sinus cavities. The maxillary and anterior ethmoid sinuses are most frequently involved in children because they are present at birth, but only the ethmoidal sinuses are pneumatized. (Refer to Pathophysiology Involved in Airway Disease earlier in this chapter.) Rhinosinusitis can be divided into acute or chronic designations. Acute rhinosinusitis (ARS) involves inflamed mucosal lining of the nasal passages and paranasal sinuses lasting as long as 4 weeks. Chronic rhinosinusitis (CRS) symptoms must persist for 12 weeks or longer (Brook, 2013). It is estimated that 5% to 10% of URIs are complicated by sinusitis (Wald et al, 2013).The common bacterial organisms responsible for ARS include Streptococcus pneumoniae, nontypable Haemophilus influenzae, Moraxella catarrhalis, and, less often, Staphylococcus aureus (Brook, 2013). The common bacteria responsible for CRS are similar to ARS. They include S. pneumonia, M. catarrhalis, and nontypable H. influenza. Chronic sinusitis may be supported by bacterial exotoxins and biofilm formation. Biofilms are a common mode of bacterial growth, resulting in multicellular communities joined together by a selfproduced extracellular matrix. They decrease the efficacy of antimicrobials by a hundredfold, allowing bacterial growth in the nose and sinuses (Rose et al, 2013). Predisposing factors for CRS include a preceding viral, bacterial, and/or fungal infection; environmental irritants; allergies; anatomic problems, including septal deviation, nasal polyps, trauma, FB, or abnormality of the ostiomeatal complex; gastroesophageal reflux; cigarette smoking; CF; primary ciliary dyskinesia, and immunodeficiencies (Brook, 2013; Rose et al, 2013). Persistent swelling of the sinonasal mucosa impairs sinus drainage. The presence of antibiotic-resistant organisms is associated with prior antibiotic therapy. The role of viruses in rhinosinusitis is not clear. Children with immunodeficiency disorders or CF are predisposed to infections with Aspergillus and Zygomycetes. Clinical Findings The duration of symptoms determines the classification of rhinosinusitis as described earlier. The history of acute sinusitis is different from an uncomplicated URI. The latter may present with a thick, yellow discharge on the third or fourth day (peaking by day 6) with no or low-grade fever followed by improvement. The history of sinusitis is either acute with high fever and purulent nasal discharge or a ―double sickening‖ in which the child experiences worsening of symptoms after initial symptoms of recovering from the URI (Wald et al, 2013). Headache, bad breath, fatigue, and decreased appetite are nonspecific symptoms and therefore not helpful in the diagnosis. CRS is also associated with intractable wheezing in children with asthma (Cherry and Shapiro, 2009). Major criteria suggestive of acute bacterial sinusitis include facial pain, facial or nasal congestion or fullness, nasal discharge, purulence or discolored postnasal drip, hyposmia or anosmia, fever, and/or purulence on intranasal examination. Minor criteria include headache, fever, halitosis, fatigue, dental pain, cough, and ear pain, pressure, or fullness (Brook, 2013). Transillumination and percussion of the sinuses are not recommended (Wald et al, 2013). Diagnostic Studies Imaging studies are not needed in sinusitis, because MRI, CT, and plain radiographs are likely to be positive for sinusitis if the patient has a cold. However, if these studies are negative for sinusitis, the child does not have sinusitis. If the child is suspected of having an orbital or CNS extension of sinusitis, then contrastenhanced CT or MRI with contrast is needed. However, both studies are complementary when the diagnosis is in doubt (Wald et al, 2013). Differential Diagnosis Viral URI, allergic rhinitis, and other causes of headache are the differential diagnoses. Remember that sinusitis may exacerbate asthma. Ethmoiditis can occur after a child is 6 months old, in contrast to frontal rhinosinusitis, which is first seen around 10 years old. Management The health provider must be cautious to not overdiagnose rhinosinusitis and subsequently indiscriminately use antibiotics (DeMuri and Wald, 2010). Chronic or recurrent rhinosinusitis may result in referral to an otolaryngologist and/or allergist. To aid the clinician in decision making about when to treat, the American Academy of Pediatrics (AAP) developed clinical guidelines for the treatment of ARS based upon three different clinical presentations (Wald et al, 2013): • The child with an URI with persistent nasal discharge or daytime cough lasting for more than 10 days without clinical improvement • The child with a URI who develops a worsening or new onset of fever, nasal discharge, or daytime cough after initial improvement • The child with a fever higher than 102.2° F (39° C) with purulent nasal discharge for at least 3 days who also has sinusitis Severe onset or a worsening course requires oral antibiotics. If the child has a persistent illness suggestiveof rhinosinusitis, antibiotics can be given or a watchful waiting for 3 days can be offered. Symptomatic pain relief with acetaminophen or ibuprofen has been shown to be helpful. The sinusitis guidelines suggest treatment with amoxicillin with or without clavulanate as a first-line treatment (Wald et al, 2013). Treatment length varies from 10 to 28 days. An alternative to this is to continue treatment for 7 days after the patient is completely free of any signs or symptoms. After starting treatment with antibiotics or watchful waiting, reassessment is needed after 72 hours. Treatment considerations include the following: • Amoxicillin at a standard dose of 45 mg/kg/day divided in two doses is the first-line treatment in communities with low incidence of nonsusceptible S. pneumoniae. In communities with more than 10% of resistant S. pneumonia, amoxicillin should be used at 80 to 90 mg/kg/day divided every 12 hours (maximum dose: 1000 mg/dose). • In patients younger than 2 years old, day care attendees, recent antimicrobial use, or in patients with moderate to severe illness, amoxicillin-clavulanate at 80 to 90 mg/day of amoxicillin component divided every 12 hours with a maximum of 2 g per dose should be prescribed. • In vomiting children, a single dose of 50 mg/kg of ceftriaxone can be given either IV or IM. In patients with allergy to amoxicillin, the type of allergic reaction determines the antibiotic: • If the child has a serious type 1 immediate or accelerated reaction, the cephalosporins cannot be used. However, if they have a non-type 1 hypersensitivity reaction, they can safely be treated with one of the third-generation, cephalosporin antibiotics—cefdinir, cefpodoxime, or cefuroxime. The management of CRS is more complicated because bacteria are generally only one of other contributing factors. Referral to an otolaryngologist is often needed. Children with complications or signs of invasive infection should be referred to the appropriate medical specialist. Surgical drainage by an otolaryngologist, treatment of allergies and control of allergic rhinitis by an allergist, or both may be necessary. Additional management considerations include the following: • Decongestants and antihistamines: There is no randomized controlled trial (RCT) to support the use of topical decongestants (Wald et al, 2013). Similarly, there are no data to support the use of either topical or oral antihistamines as an adjuvant therapy. See Chapter 25 for information on the use of decongestants and antihistamines. • Topical corticosteroids: The data for the use of topical corticosteroid in children are plagued with methodological problems; thus, a clear recommendation for topical corticosteroid use in children cannot be made. • Saline irrigation: The use of buffered isotonic saline into the nasal cavity by squeeze bottle or neti pot (in late childhood and adolescence) may be helpful, but the clinical guidelines do not support or negate the use of saline (Wald et al, 2013). Nasal saline can be used by patients to help thin secretions. • Analgesics: Comfort measures include the use of acetaminophen and ibuprofen for severe pain. Adequate hydration is important. • Diving is contraindicated with rhinosinusitis. Complications Orbital extension, inflammation, and infection from an ethmoid sinusitis in a child younger than 5 years old are the most common complications with sinusitis (Wald et al, 2013). Orbital cellulitis secondary to ethmoiditis is a serious, life-threatening complication that is a medical emergency. It is manifested by swelling and erythema of the eyelids, proptosis, decreased extraocular movements, and altered vision. Other orbital complications include periorbital or preseptal inflammation involving only the eyelid as well as orbital cellulitis. CNS inflammation and infection is another possible complication of sinusitis. Intracranial complications include Pott's puffy tumor, epidural abscess, subperiosteal abscess, brain abscess, venous thrombosis, and meningitis. The child with Pott's puffy tumor has osteomyelitis of the frontal bone, and a neurosurgical consult should be obtained. An infectious disease and otolaryngology consult is important in all patients with complications (Wald et al, 2013). Prevention Prevention of sinusitis includes good allergy management, management of gastroesophageal reflux, influenza vaccine, and relief of nasal airway obstruction. [Show More]
Last updated: 1 year ago
Preview 1 out of 74 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 15, 2021
Number of pages
74
Written in
Additional information
This document has been written for:
Uploaded
May 15, 2021
Downloads
0
Views
77


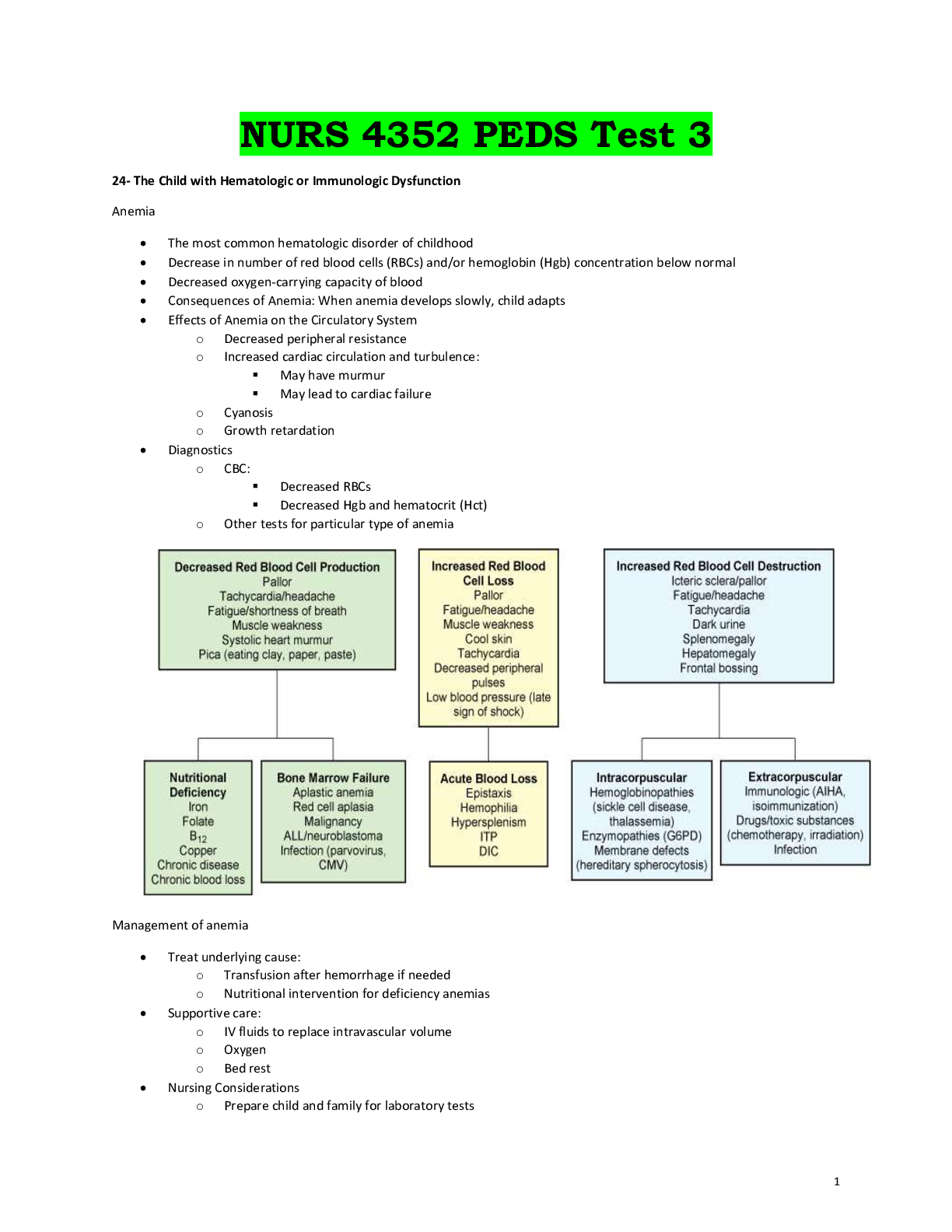





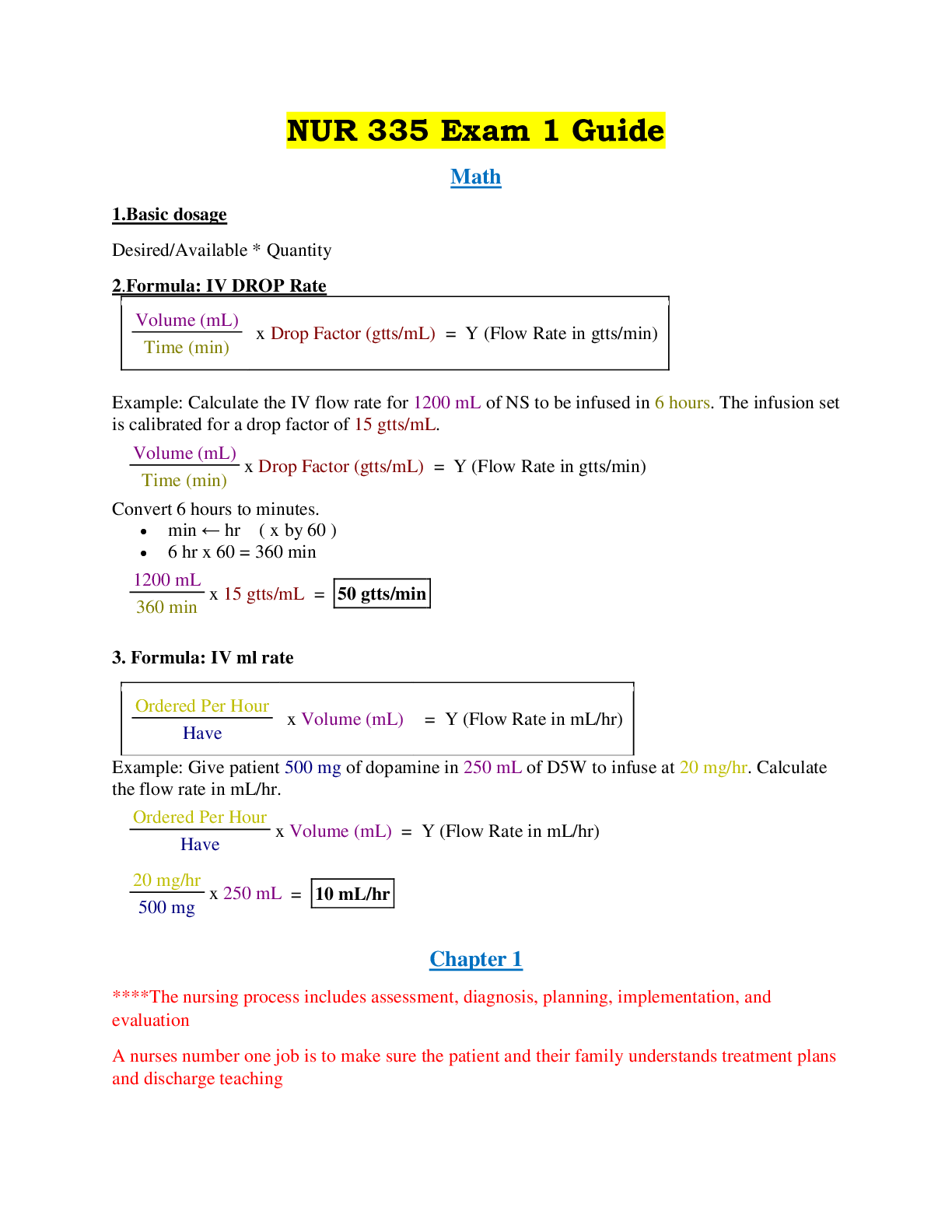







 (4).png)





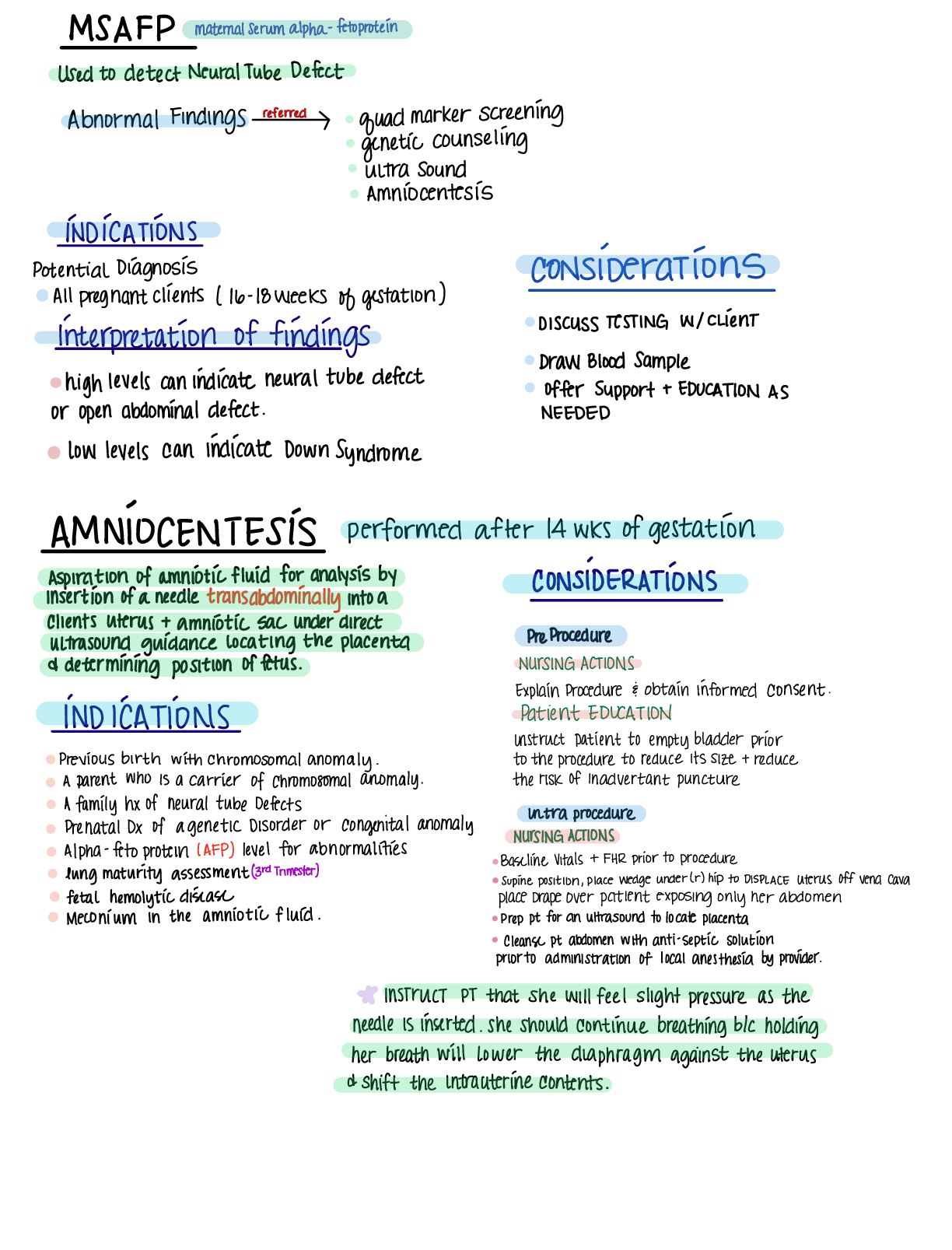

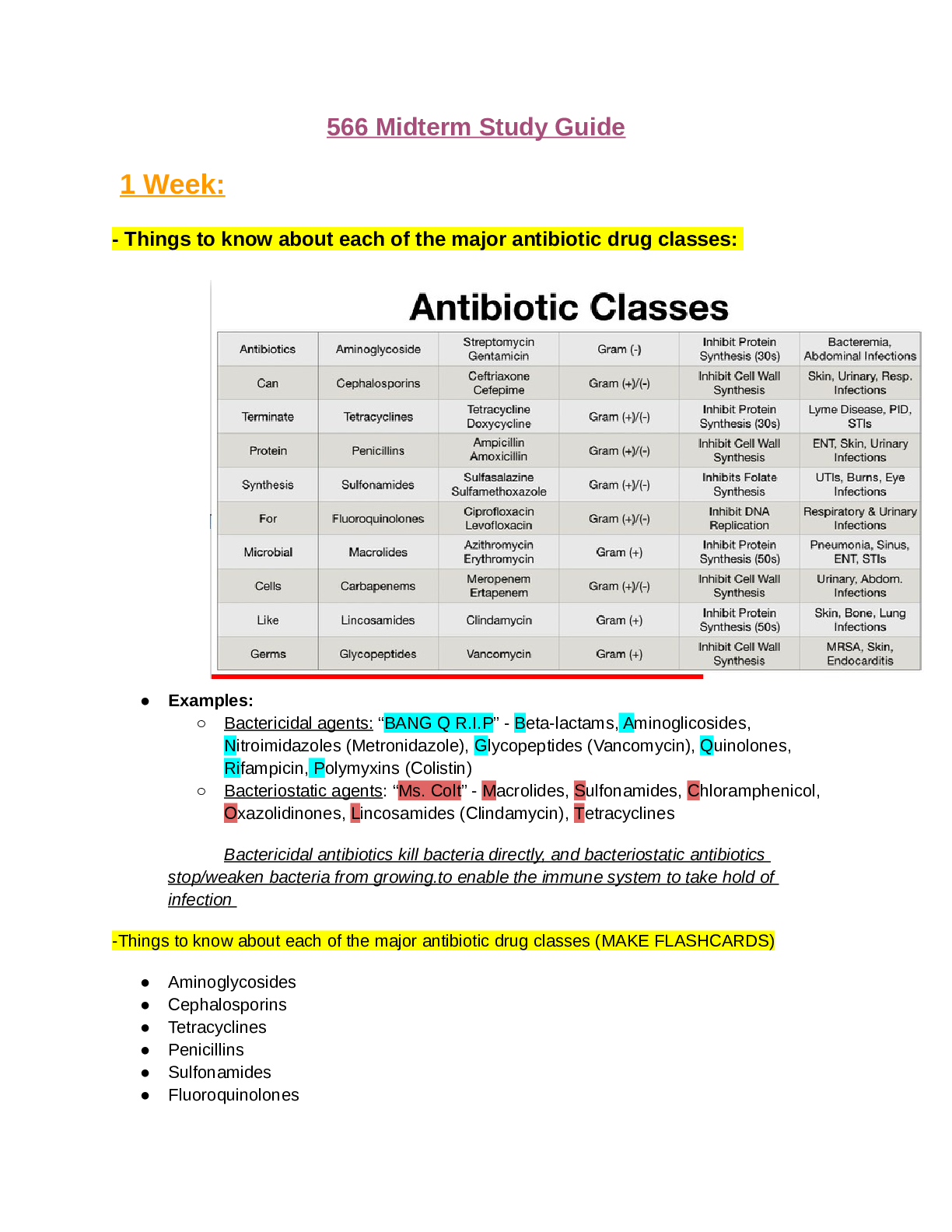





.png)



