BioChemistry > QUESTIONS & ANSWERS > Old Dominion UniversityCHEM 441PROBLEM SET 1 BIOCHEMISTRY. ( verified answers, 100% correct ) (All)
Old Dominion UniversityCHEM 441PROBLEM SET 1 BIOCHEMISTRY. ( verified answers, 100% correct )
Document Content and Description Below
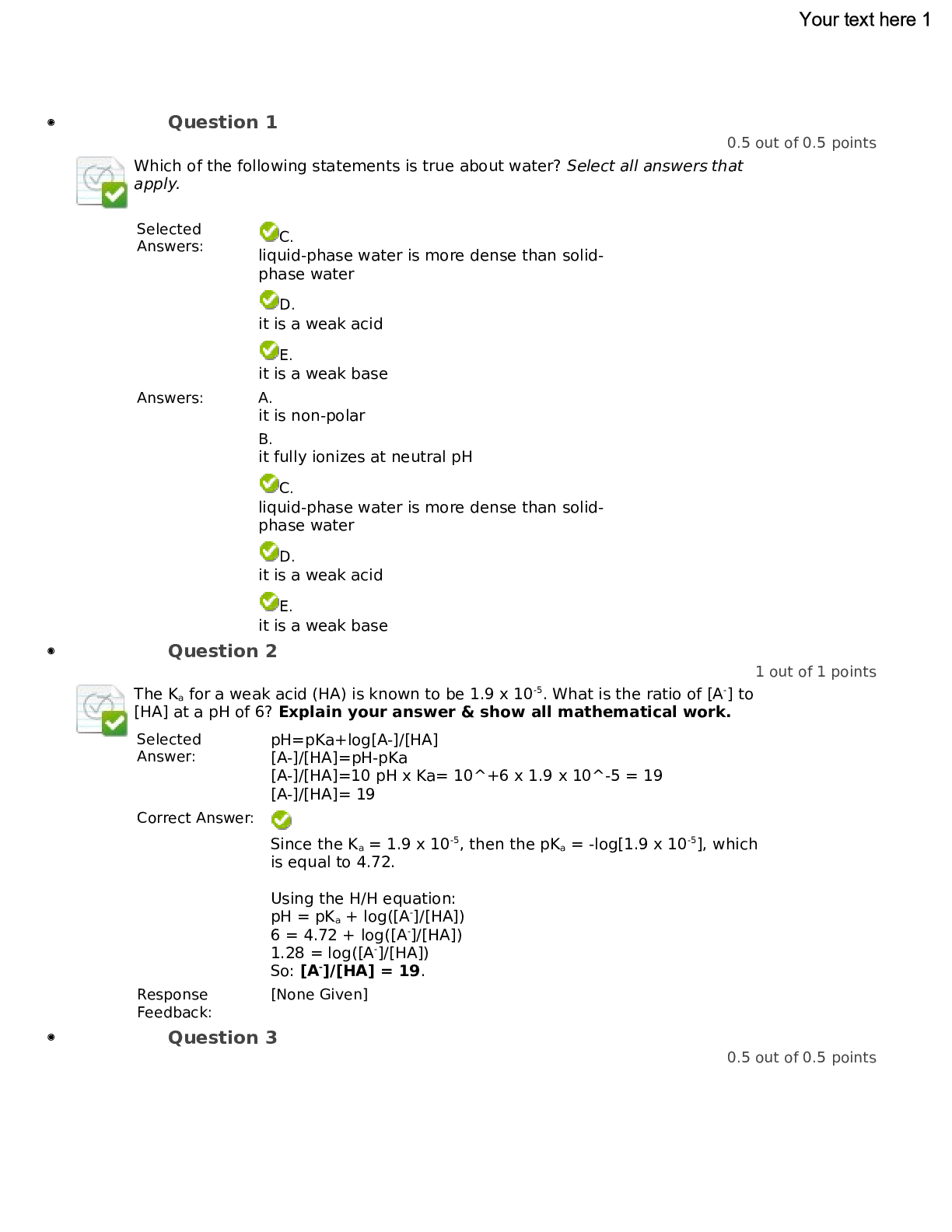
Question 1 0.5 out of 0.5 points Which of the following statements is true about water? Select all answers that apply. Selected | C.liquid-phase water is more dense than solidphase water D... . it is a weak acid E. it is a weak base Answers: A. it is non-polar B. it fully ionizes at neutral pH C. liquid-phase water is more dense than solidphase water D. it is a weak acid E. it is a weak base Question 2 1 out of 1 points The Ka for a weak acid (HA) is known to be 1.9 x 10-5. What is the ratio of [A-] to [HA] at a pH of 6? Explain your answer & show all mathematical work. Selected For questions 3-6, consider the titration curve below. The chemical species being titrated by a strong base must be a __________ , since it is capable of donating ______ proton(s). Selected Answer: E. triprotic acid; three weak base; no B. strong base; three C. monoprotic acid; one D. diprotic acid; two E. triprotic acid; three Question 4 0.5 out of 0.5 points The chemical group being titrated around the point labeled (C) in the graph is most likely a(n) ________________ (basic or acidic) group. [Blank1] Specified Answer for: Blank1 | basic Exact Match basic Response Feedback: Since the graph indicates the pKa is approximately 12.5 at point (C), then this must be a basic group, such as an amino group. Question 5 0.5 out of 0.5 points The titration curve shown in Question #3 is that of a free amino acid in aqueous solution. Based on the graph, which particular amino acid is most likely represented? Simply name the amino acid. [Blank1] Specified Answer for: Blank1 | Arginine Exact Match arginine Response Feedback: The titration curve clearly shows that the amino acid in question has three ionizable groups, due to the three distinct "steps" on the graph. So this means that the amino acid must be Tyr, Cys, Lys, His, Arg, Asp, or Glu (these are the only free amino acids with ionizable side chains). The chemical group at point (A) has a pKa of a little over 2; the chemical group at point (B) has a pKa of approximately 9; the chemical group at point (C) has a pKa of around 12.5. The only amino acid that fits this pKa profile is ARGININE. Question 6 1 out of 1 points Assume that the equilibrium represented around point (A) in the titration can generically be described as: H3A + OH- ---> H2A- + HOH What is the pH at which the ratio of [HA2-] to [H2A-] is 25:1? Show all work & clearly explain your answer. Selected Evaluation Method | Correct Answer | Case Sensitivity Exact Match | HMDYFS Question 8 0.5 out of 0.5 points How many distinct "ionizable" groups exist within this peptide? Selected Answer: | D.5 . 2 B. 3 C. 4 D .5 E. 6 Response Feedback: The ionizable groups are: The amino terminal group of His The side chain of His The side chain of Asp The side chain of Tyr The carboxy terminal group of Ser Question 9 0.5 out of 0.5 points At a pH of 6, which of the following best approximates the net charge on the peptide? Selected Answer: | B . -0.5 -1B . -0.5 C. 0 D. +0. 5 E. +1 Response Feedback: At a pH of 6, which is equal to the pKa for the imidazole side chain on His, the charges are as follows: N-terminal group is +1 His side chain is +1/2 Asp side chain is -1 Tyr side chain is 0 C-terminal group is -1 Question 10 1 out of 1 points Within this peptide, which amino acid residue is the most hydrophobic? Explain your answer. Selected . Response Feedback: [None Given] Question 11 1 out of 1 points Calculate an approximate pI (isoelectric point) for this peptide. Please use the pKa's listed within Table 3-1 of Lehninger. Show and/or explain all your work; you must be very clear about WHY you use particular pKa's in your calculation. Selected The approximate pI for this peptide is the average of the pKa's for the His side chain (6.00) and the Asp side chain (3.65), resulting in a value of 4.80. At a pH of 4.8, the His side chain is still-protonated (the pKa is more than one pH unit above the pH) and therefore contributes a +1 charge. Also at a pH of 4.8, the Asp side chain is fully deprotonated (the pKa is more than one pH unit below the pH) and therefore contributes a -1 charge. All other pKa's are well away from 4.8, thereby rendering them fully protonated or deprotonated: the amino terminus is +1; the Tyr side chain is 0; the carboxy terminus is -1. Therefore, at a pH of 4.8, the net charge on our peptide is 0. Response Feedback: [None Given] Question 12 1 out of 1 points Based on your answer for Question #11, would you categorize this peptide as "acidic" or "basic"? Explain. Selected Answer: : | This is definitely an acidic peptide because it's pI is well below neutral pH (7). This means that the peptide loses more than half of its dissociable protons at low pH values, meaning it contains amajority of acidic groups. Response Feedback: [None Given] Question 13 0.5 out of 1 points At a pH of 10, would you expect this peptide to be retained for a longer time within an anion exchange column or a cation exchange column? Clearly explain your reasoning. Selected It would be retaining it for a longer time because the peptide will be negatively charged and the column matrix will be positively charged. Correct Answer: At a pH of 10, most of this peptide's ionizable groups are either negatively-charged or neutral. We would therefore expect the peptide to be retained longer within an anion exchange column. The negatively-charged groups within the peptide would bind electrostatically to the positivelycharged immobile phase of the column. Response Feedback: which column? Question 14 0.5 out of 0.5 points The amino acid residues commonly found within a β turn are: Selected Pro & Gly. Answers: | A.Ala & Gly. B. two Cys. C. hydrophobic. D. Pro & Gly. E. those with ionized R groups. Question 15 1.5 out of 1.5 points Cellular proteins are oftentimes post-translationally modified. Choose oneof the following PTMs: N-linked glycosylation, phosphorylation, ubiquitination, or GPIanchor. Clearly indicate your choice, then address the following: (a) How is the PTM attached to the protein of interest? At which amino acid residue(s)? What enzyme(s) is involved, if any? (b) Is the PTM relatively stable or highly dynamic? Explain. How does the PTM become detached from the protein of interest? What enzyme(s) is involved, if any? (c) What is the function of the PTM? Provide one specific example. Selected (a) Phosphorylation is done by kinases. Kinases attaches to phosphate groups which attaches to serines and threonines. (b) Phospholate proteins are generally activated and are highly dynamic. The phosphate group can be removed by a phosphatase. (c) Phosphorylation and De-phosphorylation of proteins can be see in signal transduction of the adrenaline receptor. Correct Answer: Response Feedback: | Answers will obviously vary, based on PTM selected. [None Given] Question 16 0.5 out of 0.5 points Disulfide linkages: Selected are covalent bonds. A. are covalent bonds. | Answers:B. are a type of electrostatic interaction. C. occur between methionine residues. D. can be disrupted by SDS. E. can only occur within the same polypeptide chain. Question 17 0.5 out of 0.5 points The idea that primary sequence determines tertiary structure first came from experiments in the 1950's about: Selected the renaturation of RNaseA (ribonuclease A). A. | Answers:the renaturation of RNaseA (ribonuclease A). B. the denaturation of lysozyme. C. the 3D structure of lysozyme. D. the role of Hsp70 in protein folding. E. the role of PDI (protein disulfide isomerase) in protein folding. Question 18 1 out of 1 points Questions #18 & 19 refer to the following information. Altered conformations of the prion protein (PrP) are implicated in the infectivity and/or pathogenesis of Scrapie-like diseases, such as "mad cow disease" and Creutzfeldt-Jacob Disease (CJD). Below are ribbon diagrams of two different conformations of PrP: Which of the conformations illustrated above (a or b) is more likely PrPc(the normal cellular form of PrP) and which is more likely PrPSc (the pathogenic form of PrP)? Why? Selected Normal Cellular form PrP is a because it is primarily alpha-helical. Pathogenic form of PrP is b because it is primarily folded in betasheets. Correct Response Feedback: [None Given] Question 19 0.5 out of 0.5 points The red arrows within the (b) conformation are best categorized as: Selected | E. antiparallel beta strands | A.alpha helices B. beta-turns C. tertiary structure D. parallel beta-strands E. antiparallel betastrands Response Feedback: This rendering is precisely the way that ribbon diagrams convey the secondary structure of a beta sheet. A flat, ribbon-like appearance indicates a beta-strand. The direction of the arrows tells us that the two (red) strands are in an anti-parallel orientation to each other. Question 20 0.5 out of 0.5 points Questions #20-23 refer to the figure shown below. Approach each question independently. Figure Legend: Tagging schematic. The figure shows various steps involved in the identification of interacting proteins by using an epitope-tagging strategy. The cDNA of interest is first cloned into a vector that provides an epitope tag. This is followed by transfection of the tagged "bait" into the cell of interest. The cells are then lysed and the lysates purified by affinity purification using an antibody against the epitope. Proteins bound specifically to the bait protein are eluted by competitive elution using a peptide that encodes the epitope. The proteins are then resolved by gel electrophoresis followed by mass spectrometric identification. STEP 1: Epitope-tagged protein (encoded by cDNA) STEP 2: Transfect into cells STEP 3: Lyse cells STEP 4: Affinity-based purification using immobilized antibody against the epitope STEP 5: Competitive elution with peptide encoding the epitope STEP 6: Gel electrophoresis STEP 7: Excise bands and analyze by mass spectrometry Question #20: According to the gel (Step 6), which protein has the highest molecular weight? Selected Answers: | A.Protein A B. Protein B C. Protein C D. Protein D E. YFP Question 21 1 out of 1 points Are the bands indicated in Step 6 (SDS-PAGE) likely visualized by western blot or by a general protein stain (such as Coomassie or silver)? Explain your answer. Selected In step 6, it is visualized with a general stain because the proteins A, B, C and D are not known and the antibodies that fight these proteins are not available. Correct Answer: | Since all proteins in the complex can be "seen" on the gel, then it must not be a western, but rather a general protein stain. If we wereseeing a western, then we would only have seen one of the many bands shown, as an antibody against just one of those proteins would have been used. Response Feedback: [None Given] Question 22 1 out of 1 points Step 7 states "Excise bands and analyze by mass spectrometry." (A) Following excision of the bands (simply cutting each band out of the gel), briefly describe each step, in order, that must be taken prior to analysis in the mass spectrometer. (B) What's the big experimental question that the researchers hope to answer following Step 7? Selected (A) Deleted bands must first be treated to get rid of background contaminating proteins. This can be done through the use of glassware, gloves and a wash of 80% acetic acid and then washed with water, followed by methanol and lastly with more water. (B) I believe that researchers want to be able to identify the unknown proteins A,B,C and D that interact with YFP 2. digest with protease (like trypsin) or CNBr 3. separate peptides generated in Step 2 via chromatography (reverse-phase HPLC) 4. sequence one (or several) peptides, from Step 3, by mass spec (B) The big question: "What proteins specifically interact with YFP?" The mass spec analysis will allow them to IDENTIFY the proteins (A, B, C, D) that associate with YFP. Response Feedback: [None Given] Question 23 0.5 out of 0.5 points Following Step 5 ("competitive elution with peptide encoding the epitope"), it appears that the YFP/protein complex has dissociated -- all proteins are drawn floating freely. Which of the following is the most likely explanation for dissociation of the protein complex? Selected Treatment of the eluate (Step 5) with sodium dodecyl sulfate, in preparation for loading onto the gel (Step 6) Answers: | A.Treatment of the eluate (Step 5) with beta-mercaptoethanol, in preparation for loading onto the gel (Step 6) B. Treatment of the eluate (Step 5) with dithiothreitol, in preparation for loading onto the gel (Step 6) C. Treatment of the eluate (Step 5) with sodium dodecyl sulfate, in preparation for loading onto the gel (Step 6) D. Exposure of the complex to free peptide encoding the epitope (in other words, Step 5, the elution process itself) E. Placing the eluate (Step 5) on ice, prior to loading onto the gel (Step 6) Question 24 1.5 out of 1.5 points A colleague has successfully purified the enzyme sphingomyelinase from bovine brain. She provides you with the following incomplete purification table: Per your colleague's request, you agree to calculate: (A) the final specific activity, (B) the final % yield, and (C) the final fold-purification. Show all work and/or provide explanations, clearly label each part (letter); indicate proper units where appropriate. Selected Answer: (A) Specific activity following isoelectric focusing is calculated by dividing that step's total activity (420 nmol/min) by that step's total protein (0.03 mg). This gives a value of 14,000 nmol/min/mg for the specific activity. (B) % yield, following isoelectric focusing, is calculated by dividing that step's total activity (420 nmol/min) by the initial total activity (51,700 nmol/min) and multiplying by 100 to get a percent. This gives a value of 0.81 %. (C) Purification-fold, following isoelectric focusing, is calculated by dividing that step's specific activity (14,000 nmol/min/mg) by the initial specific activity (0.58 nmol/min/mg). Note the initial specific activity is calculated by dividing the initial total activity (51,700 nmol/min) by the initial total protein (88,625 mg). The final answer is 24,138-fold. Response Feedback: [None Given] Question 25 0.5 out of 0.5 points For the table shown in Question #24, the purification step labeled "40-60% (NH4)2SO4" is most likely a(n): Selected salting in/out step. A. salting in/out step. | Answers:B. cation exchange chromatography. C. anion exchange chromatography. D. hydrophobic interaction chromatography. E. size exclusion chromatography. Question 26 0.5 out of 0.5 points For the table shown in Question #24, the purification step labeled "CMsepharose" is most likely a(n): Selected Answer: | B.cation exchange chromatography. salting in/out step.B. cation exchange chromatography. C. anion exchange chromatography. D. hydrophobic interaction chromatography. E. size exclusion chromatography. Question 27 0.5 out of 0.5 points For the table shown in Question #24, the purification step labeled "Gel filtration" is most likely a(n): Selected size exclusion chromatography. salting in/out step. B. cation exchange chromatography. C. anion exchange chromatography. D. hydrophobic interaction chromatography. E. size exclusion chromatography. [Show More]
Last updated: 1 year ago
Preview 1 out of 16 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 12, 2021
Number of pages
16
Written in
Additional information
This document has been written for:
Uploaded
Jun 12, 2021
Downloads
1
Views
69

.png)
.png)
.png)

.png)
.png)




.png)




.png)
.png)
.png)










.png)

