Chemistry > Lab Report > Danbury High School - CHEMISTRY 101Sodium carbonate lab (All)
Danbury High School - CHEMISTRY 101Sodium carbonate lab
Document Content and Description Below
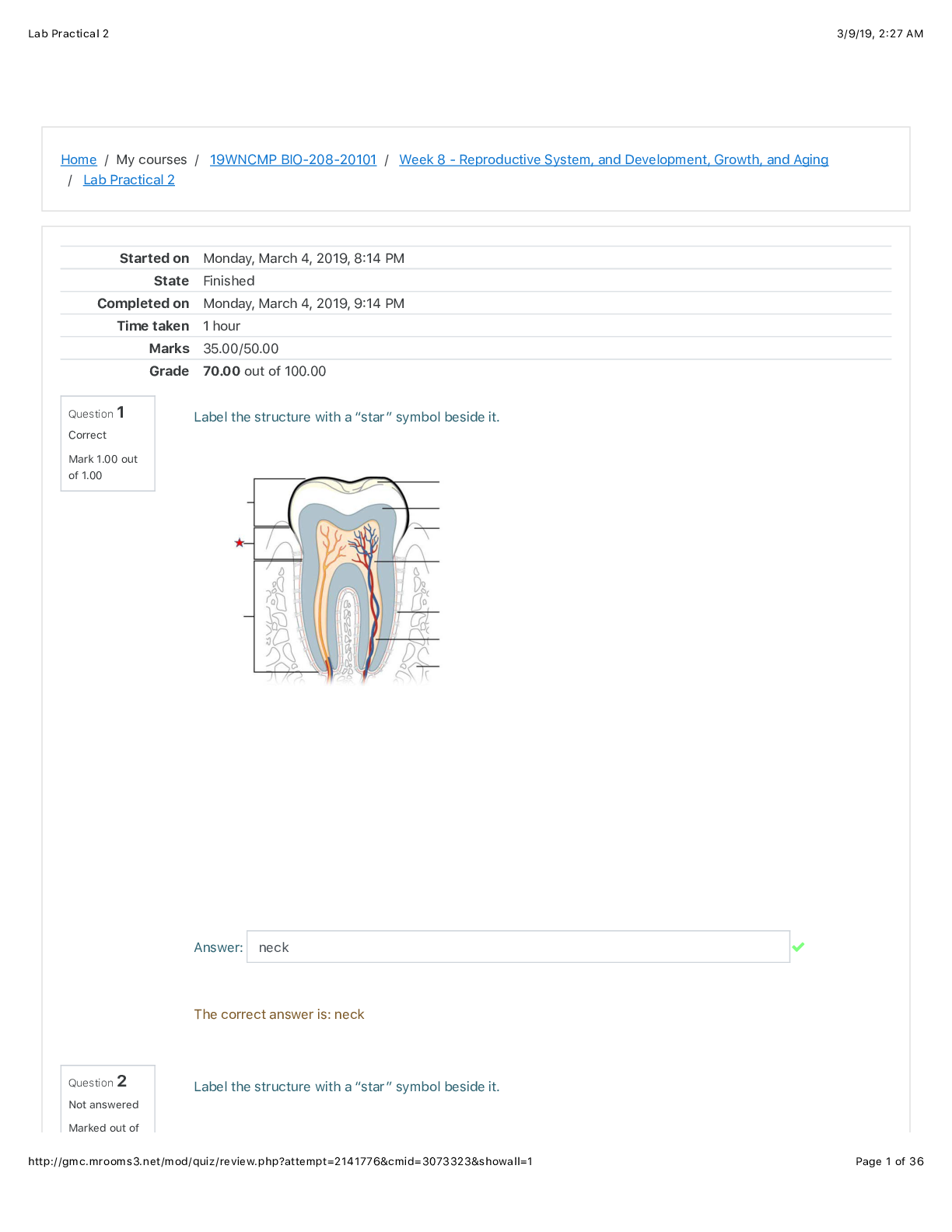
Stoichiometry of the reaction of Sodium Carbonate with hydrochloric acid http://www.dlt.ncssm.edu/core/Chapter6-Stoichiometry/Chapter6- Labs/NaHCO3_HCl_stoich_lab.htm Purpose: • To calculate th... e theoretical (expected) yield of product in a reaction. • To weigh the actual (experimental) mass of product collected in a reaction. • To calculate the % yield of the chemical reaction. • To practice making conversions and taking accurate measurements. Background information: Stoichiometry will be used to investigate the amounts of reactants and products that are involved. The word stoichiometry is derived from two Greek words: stoicheion (meaning “element”) and metron (meaning “measure”). Stoichiometry is an important field of chemistry that uses calculations to determine the quantities (masses, volumes) of reactants and products involved in chemical reactions. It is a very mathematical part of chemistry. In real-life situations the number of moles of reactants available for a chemical reaction is not always exactly the same as the ratio of moles in the balanced equation. One of the reactants will run out before the other reactant. For instance, when a candle burns in an open room, the wax in the candle will run out long before all the oxygen in the air will be used up. On the other hand, put the candle in a closed jar, and the oxygen will be used up quickly, leaving the wax left over. When one of the reactants is used up in a situation like this, the reaction will stop, and no more product will be made. The reactant that gets used up first, and therefore limits the amount of product that can be made, is called the limiting reactant. The reactant that is left over is called the excess reactant. It is often difficult as well as impractical to combine just the right amount of each reactant that is required for a particular reaction to occur. In the lab as well as in industrial processes, reactions are rarely carried out with exactly the required amount of each reactant. In most reactions, one or more reactants are in excess. Given this fact, this experiment is designed so that only one of the reactants will be completely used up. This is called the limiting reactant because it limits the amount of products formed. Since the other reactant will have a quantity remaining, it is called the excess reactant. One of your tasks will be to determine which of your reactants is limiting and which is in excess. For a chemical reaction to happen the reactant molecules must collide with one another. All collisions do not lead to a chemical reaction. Only those collisions with proper orientation (angle of collision) and force of collision can cause the existing bonds to break and allow new bonds to [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages
Instant download
.png)
Instant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 18, 2021
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Apr 18, 2021
Downloads
0
Views
48

.png)



.png)




.png)







 (1).png)





.png)


