Chemistry > QUESTIONS & ANSWERS > CHM 2290 Analytical Chemistry lab - Wayne State University. (All)
CHM 2290 Analytical Chemistry lab - Wayne State University.
Document Content and Description Below
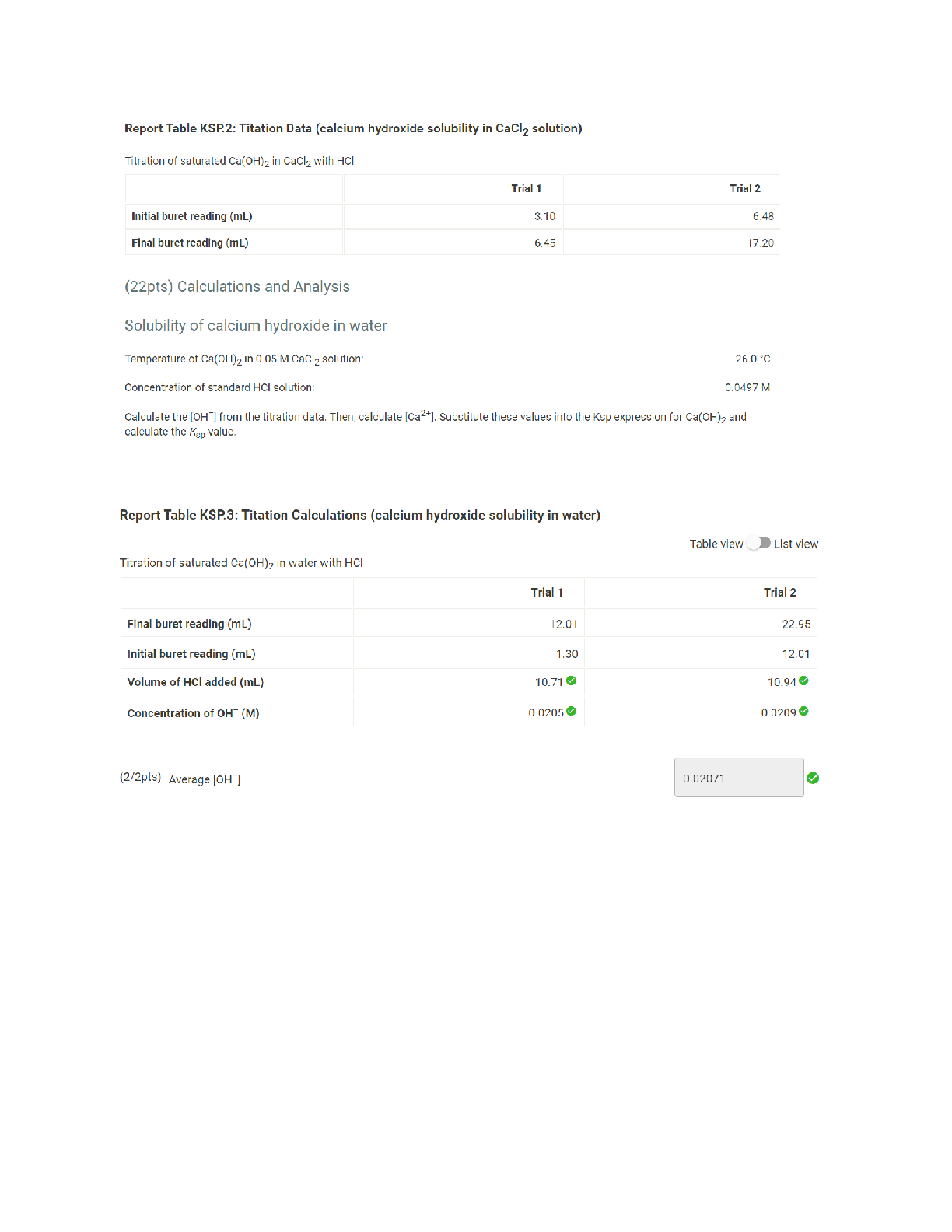
Report Table KSP.2: Titation Data (calcium hydroxide solubility in CaCl2 solution) Titration of saturated Ca(OH)2 in CaCl2 with HCI Trial 1 Trial 2 Initial buret reading (mL) 3.10 6.48 Final buret rea... ding (mL) 6.45 17.20 (22pts) Calculations and Analysis Solubility of calcium hydroxide in water Temperature of Ca(OH)2 in 0.05 M CaCl2 solution: 26.0 .C Concentration of standard HCI solution: 0.0497 M Calculate the [OH"] from the titration data. Then, calculate [Ca2+]. Substitute these values into the Ksp expression for Ca(OH)2 and calculate the Ksp value Report Table KSP.3: Titation Calculations (calcium hydroxide solubility in water) Table view List view Titration of saturated Ca(OH)2 in water with HCI Trial 1 Trial 2 Final buret reading (mL) 12.01 22.95 Initial buret reading (mL) 1.30 12.01 Volume of HCI added (mL) 10.71 10.94 Concentration of OH" (M) 0.0205 0.0209 (2/2pts) Average [OH ] 0.02071 Equilibrium concentrations of Cast and OH Ca(OH)2 [Ca2+] + [OH ] Initial 0.05 x 1.00 Change +XV +2x Equilibrium 0.05+x 2 0.10+2x 2 (-1 pts) Incorrect. Consider if any Cast is present prior to the dissolution of Ca(OH)2 in pure water. (-1 pts) Incorrect. Consider if any significant amount of OH is present prior to the dissolution of Ca(OH)2 in pure water. (-1 pts) Incorrect. Consider the initial concentration and change in [Ca2+] to write an expression for the concentration of [Ca2+] at equilibrium. (-1 pts) Incorrect. Consider the initial concentration and change in [OH"] to write an expression for the concentration of [OH ] at equilibrium. (2/2pts) Concentration of Ca2+ (M) 0.0104 (2/2pts) Value of Ksp 4.46x10^-6 V (2/2pts) What is the molar solubility of Ca(OH)2 in pure water? 0.0104 (20pts) Solubility of calcium hydroxide in 0.05 M CaCl2 Temperature of Ca(OH)2 in 0.05 M CaCl2: 24.9 .C Concentration of standard HCI solution: 0.0497 M Calculate the [OH"] from the titration data and the stoichiometry of the dissolution process to determine the molar solubility of Ca(OH)2 in 0.05 M CaCl2. Report Table KSP.5: Titation Calculations (calcium hydroxide solubility in CaCl2 solution) Table view List view Titration of saturated Ca(OH)2 in CaCl2 with HCI Trial 1 Trial 2 Final buret reading (mL) 6.45 17.20 Initial buret reading (mL) 3.10 6.48 Volume of HCI added (ml) 3.35 10.72 Concentration of OH- (M) 0.00666 0.0213 Report Table KSP.6: ICE Table: Solubility of Ca(OH)2 in 0.05 M CaCI2 Table View 7_) List View Equilibrium concentrations of Ca2+ and OH" Ca(OH)2 .2 [ca2'1 + [OH'l Initial — 0.050 1.006) Change 7 —x (9 —2x 6) Equilibrium — 0.05-x® (110-2): ® (-1 pts) Incorrect. Consider if any significant amount of OH" is present prior to the dissolution of Ca(OH)2 in pure water. (-1 pts) Incorrect. Consider the reaction forthe dissolution of Ca(0H)2. (-1 pts) Incorrect. Consider the initial concentration and change in [Ca2+] to write an expression for the concentration of [Ca2+] at equilibrium. (—1 pts) Incorrect. Consider the initial concentration and change in [OH'] to write an expression for the concentration of [OH—l at equilibrium. (0/2pts) Concentration of Ca2+ (M) 0.00699 X (-2 pts) Incorrect. Consider the starting concentration of Cast and if this value changed significantly throughout the experiment. (2/2pts) What is the molar solubility of Ca(OH)2 in 0.05 M CaCl2? 0.00699 3. Explain how the presence of Caclz affects the molar solubility of Ca(OH)2. Use data from your experiment tojustify your answer. (pending/4pts) 4. Based on your data, is Ca(OH)2 soluble, insoluble, or sparingly soluble? Explain why you made your decision using your value of Ksp in this lab. [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 05, 2023
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Apr 05, 2023
Downloads
0
Views
98