Portage Learning Chem 210 - Module 1 exam. 2022
Document Content and Description Below
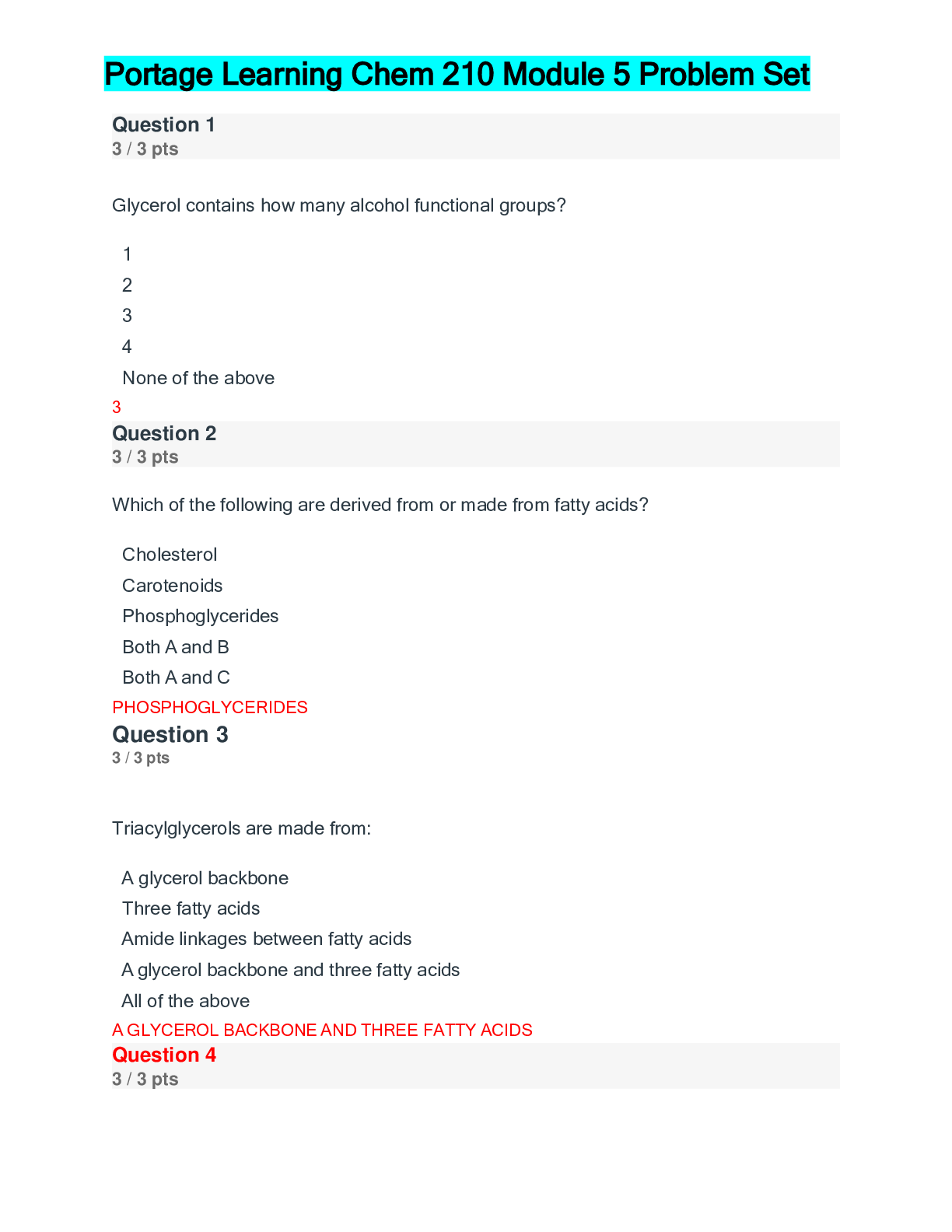
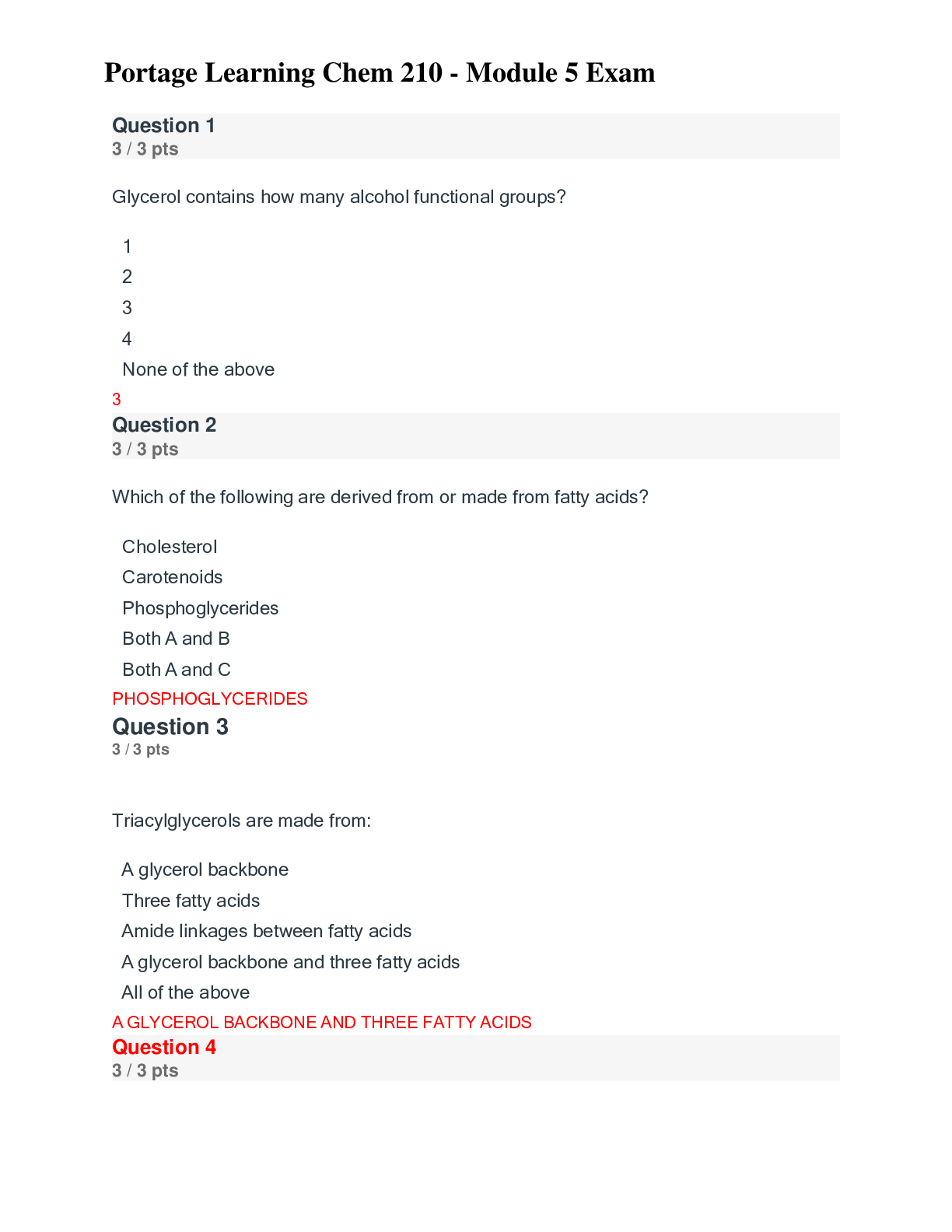
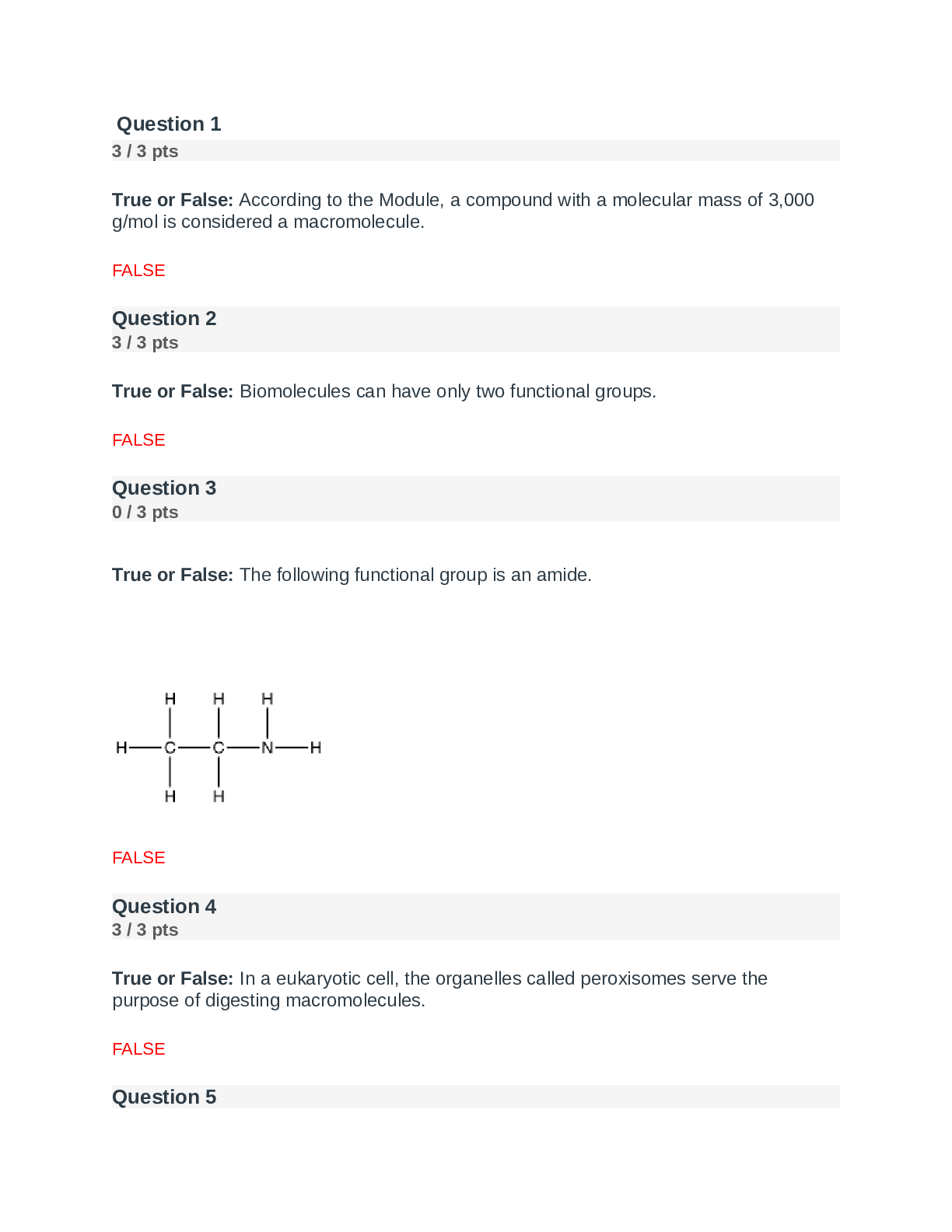
Question 1 3 / 3 pts True or False: According to the Module, a compound with a molecular mass of 3,000 g/mol is considered a macromolecule. FALSE Question 2 3 / 3 pts True or False: Biomolecule... s can have only two functional groups. FALSE Question 3 0 / 3 pts True or False: The following functional group is an amide. FALSE Question 4 3 / 3 pts True or False: In a eukaryotic cell, the organelles called peroxisomes serve the purpose of digesting macromolecules. FALSE Question 5 Portage Learning Chem 210-Module 1 Exam. 3 / 3 pts True or False: Carbon dioxide has a linear molecular shape AND has a bond angle of 120o. FALSE Question 6 3 / 3 pts Of the following, ____________ is not a property of carbon. Correct! forming ionic bonds forming cyclic structures forming multiple bonds forming bonds with oxygen bonding in long chains FORMING IONIC BONDS Question 7 3 / 3 pts Which of the following is an element not typically found in living organisms? Correct! Br Ca H S Na Br Question 8 3 / 3 pts _____________ is a functional group found in carbohydrates. Esters Thiols Carboxylic acids Correct! Alcohols Amines ALCOHOLS Question 9 3 / 3 pts The following functional group is an example of _________. Correct! Ketone Thiol Amide Benzene Aldehyde KETONE Question 10 3 / 3 pts A ___________ is a chemical formula that does NOT show the C-H and C-C bonds. Covalent formula Line bond formula Empirical formula Kekule’ structure Condensed formula CONDENSED FORMULA Question 11 3 / 3 pts In biomolecular structure, if two macromolecules interact it is called a _______. Monomer Dimer Macromolecular structure Supramolecular complex Quasimer complex SUPRAMOLECULAR COMPLEX Question 12 3 / 3 pts An organic compound with this structure, C=C, contains a ________ functional group. Alkane Alkene Alkyne Alcohol Both b and d ALKENE Question 13 3 / 3 pts Scientists refer to ________ as the “super glue” of the chemistry. C Co Na N O C Question 14 3 / 3 pts What energy molecule is produced via respiration?Correct! ATP Creatine DNA RNA mitochondria ATP Question 15 3 / 3 pts What would be the approximate size of a human cell? a. 0.1 inch b. 1 nm c. 10 nm d. 10 �μm e. 10 cm a. b. c. d. e. D. 10um Question 16 0 / 3 pts For butane, there are________ carbon and ________hydrogen atoms. A. 4, 8 B. 4, 10 C. 8, 4 D. 8, 10 E. Both b and d B. 4,10 Question 17 3 / 3 pts For an organic compound, which structure is the most efficient to draw? Condensed Kekule’ Line bond Structural None of these LINE BOND Question 18 3 / 3 pts When writing an organic functional group, scientists often write an “R” as part of the structure. What does the R indicate? Argon Rest of the molecule Routine carbon atom A string of carbon atoms None of the above REST OF THE MOLECULE Question 19 3 / 3 pts According to the module, the study of NON-carbon compounds is referred to as____. Organic chemistry Inorganic chemistry Biochemistry Environmental chemistry None of the above. INORGANIC CHEMISTRY Question 20 3 / 3 pts The ___________ functional group was NOT discussed in this module. Alcohol Amine Aromatic Thiol All of these were discussed. ALL OF THESE WERE DISCUSSED Question 21 3 / 3 pts Which number would be closest to the approximate number of ribosomes in an E. coli cell? A. 1 B. 25 C. 250 D. 25,000 E. Both c and d D. 25,000 Question 22 3 / 3 pts Which of the following would most likely have a cell wall? Marigold flower cells Deer cells Heart cells Human red blood cells All of the above MARIGOLD FLOWER CELLS Question 23 3 / 3 pts Eukaryotes have molecules that provide a protective structure. This network, which is found in all eukaryotes, is called the _____________. Cell wall Cytoskeleton Cytosol Cytoplasm None of the above CYTOSKELETON Question 24 3 / 3 pts The molecule linked to the medical condition of gout is __________. Uric acid Luciferin Luciferase Glucose Glycine URIC ACID Question 25 3 / 3 pts About how many different elements are found in living organisms? 9 30 50 68 92 30 Question 26 5 / 5 pts (Short response) Explain the common similarity in all prokaryotic and eukaryotic cells. Explain the similarity in at least two sentences. The fundamental similarity is that each cell type has a plasma membrane that separates life from non-life. The plasma membrane acts as a barrier to most molecules but does have proteins that permit select molecules to cross via proteins (transporters). The plasma membrane permits the cell to have a different composition of molecules inside the cell than out and defines a space for life to occur. Question 27 5 / 5 pts (Short response) Would a scientist be more likely to find an element such as O in a biomolecule, or W? Explain which she would more likely find in a biological molecule and give specific physical/chemical properties. She would more likely find O. Smaller elements are preferred. In the case of O, it is found in water, so it would be highly concentrated. Smaller elements with smaller atomic shells are favored because they can form stable covalent bonds. Strong bonds form by significant overlap of atoms, such as carbon and hydrogen permitting them to share electrons. The bond that forms requires a substantial amount of energy to break, which allows the bonds to withstand insults, such as mechanical and thermal stresses. This bond strength is good news for living organisms. Stable bonds allow cells to form, hair to grow, and skin to protect against abrasion. Larger elements, such as W, tend to form ionic compounds–not covalent. Question 28 5 / 5 pts (Short response) Describe at least three properties of carbon that permit it to be the basis of life. 1. Carbon can form strong covalent bonds with a variety of different elements. 2. Besides, carbon can form double and triple bonds with other carbon atoms and other elements (N and O). 3. Carbon can form long chains by forming numerous carbon-carbon bonds; we call these large molecules polymer. 4. Lastly, carbon can form cyclic structures, which are also called ring compounds. Question 29 5 / 5 pts (Short response) What the definition of inorganic chemistry? How does it differ from organic chemistry? Inorganic chemistry is the study of all other elements, but carbon. Organic chemistry is the study of carbon-based compounds both in living and non-living organisms. There is no need to have separate sub-disciples for organic and inorganic chemistry, but for historical and organizational reasons, the difference exists. Question 30 5 / 5 pts (Short response) Biochemists study the communication within and among organisms. According to the module, what are two other aspects of living organisms that biochemists study? In the module, biochemists also study the structure and function of biomolecules and the chemical reactions of organisms. [Show More]
Last updated: 1 year ago
Preview 1 out of 11 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jul 12, 2022
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Jul 12, 2022
Downloads
0
Views
45