Portage Learning Chem 210 - Module 2 exam,Graded A.
Document Content and Description Below
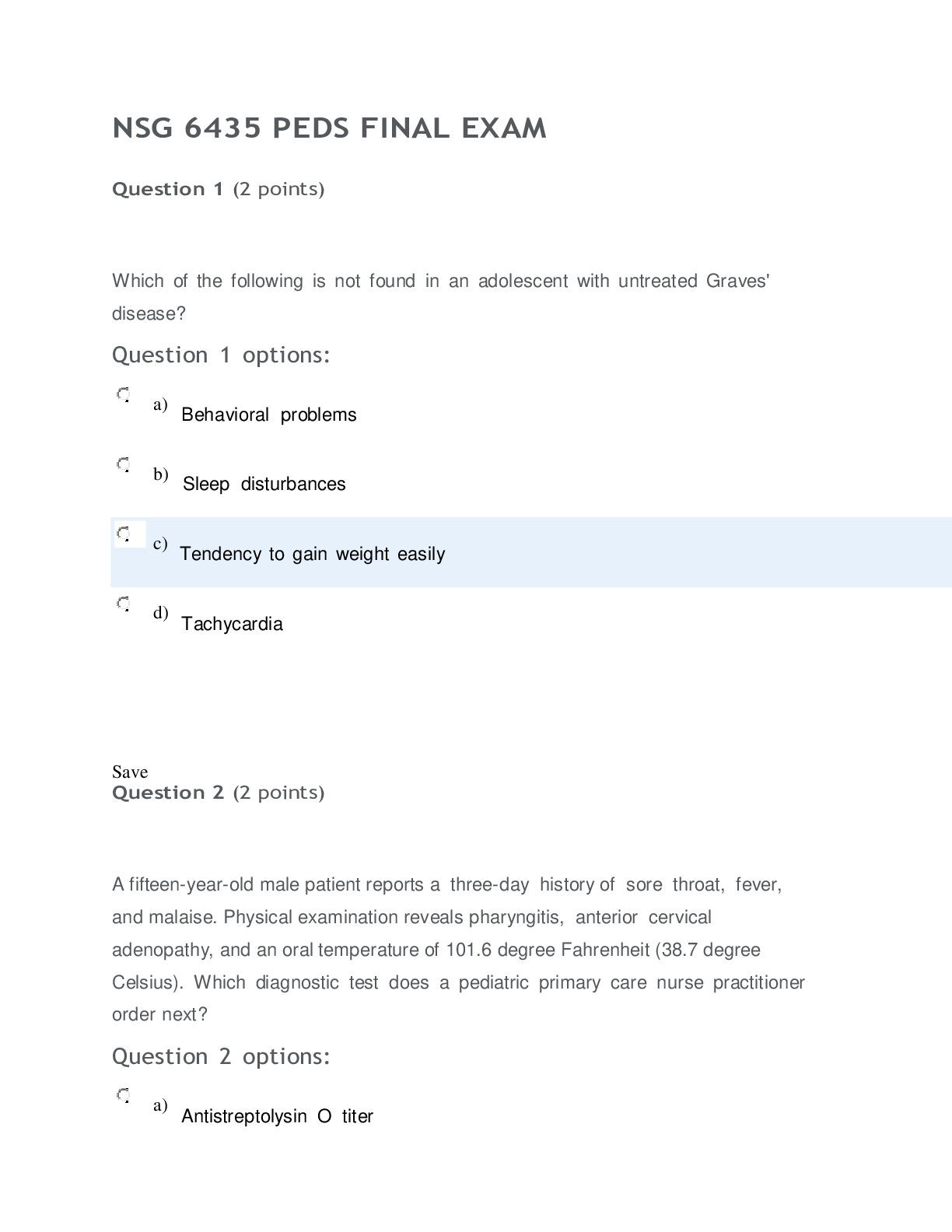
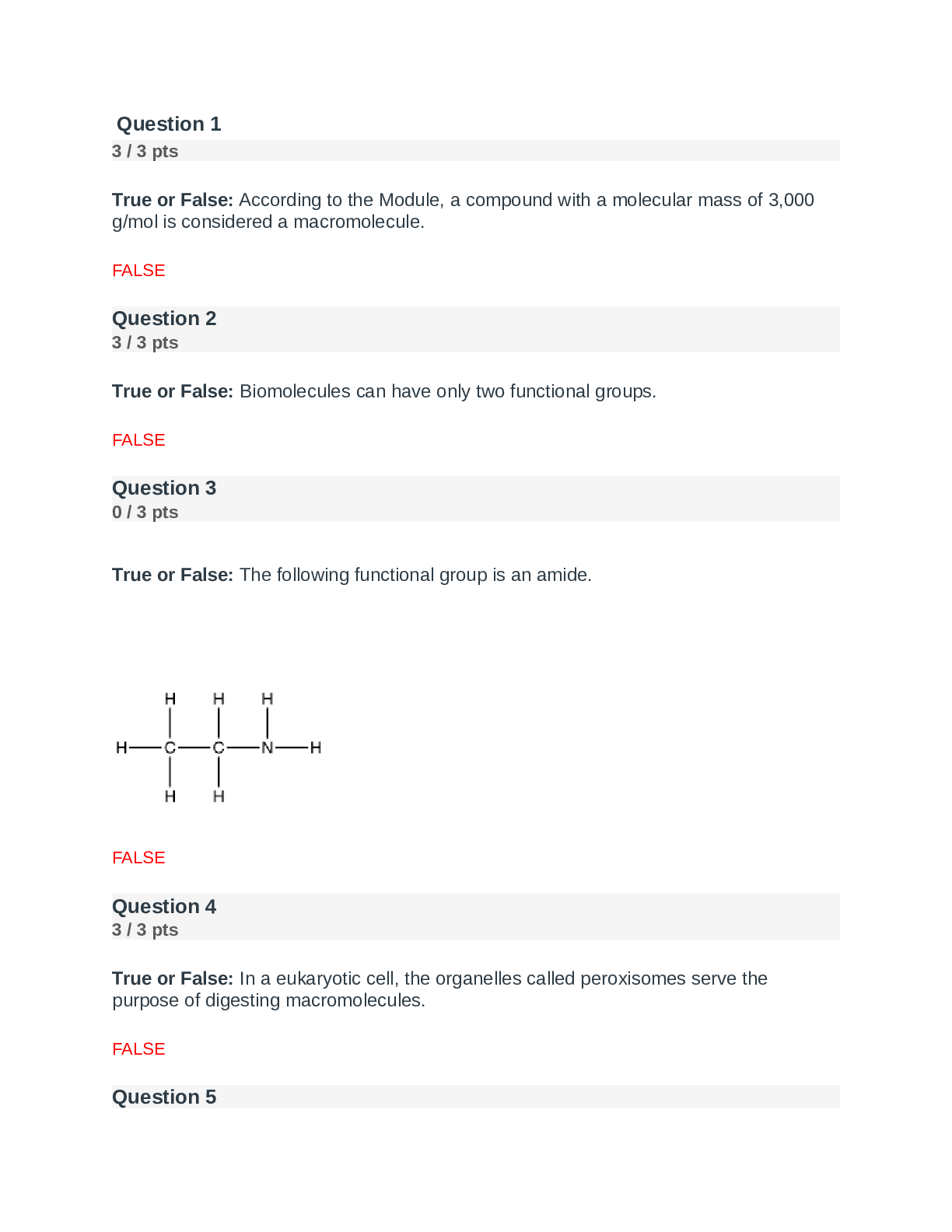
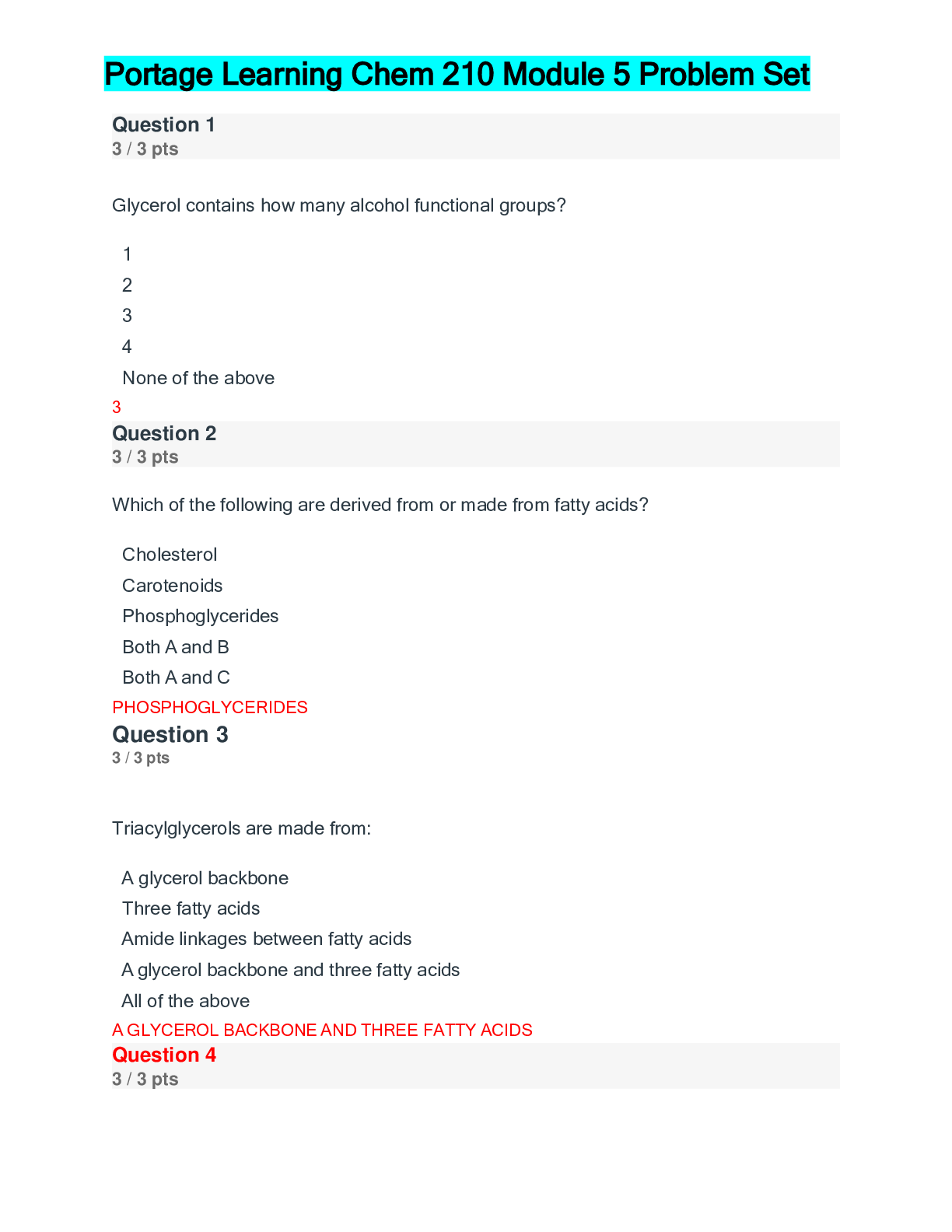
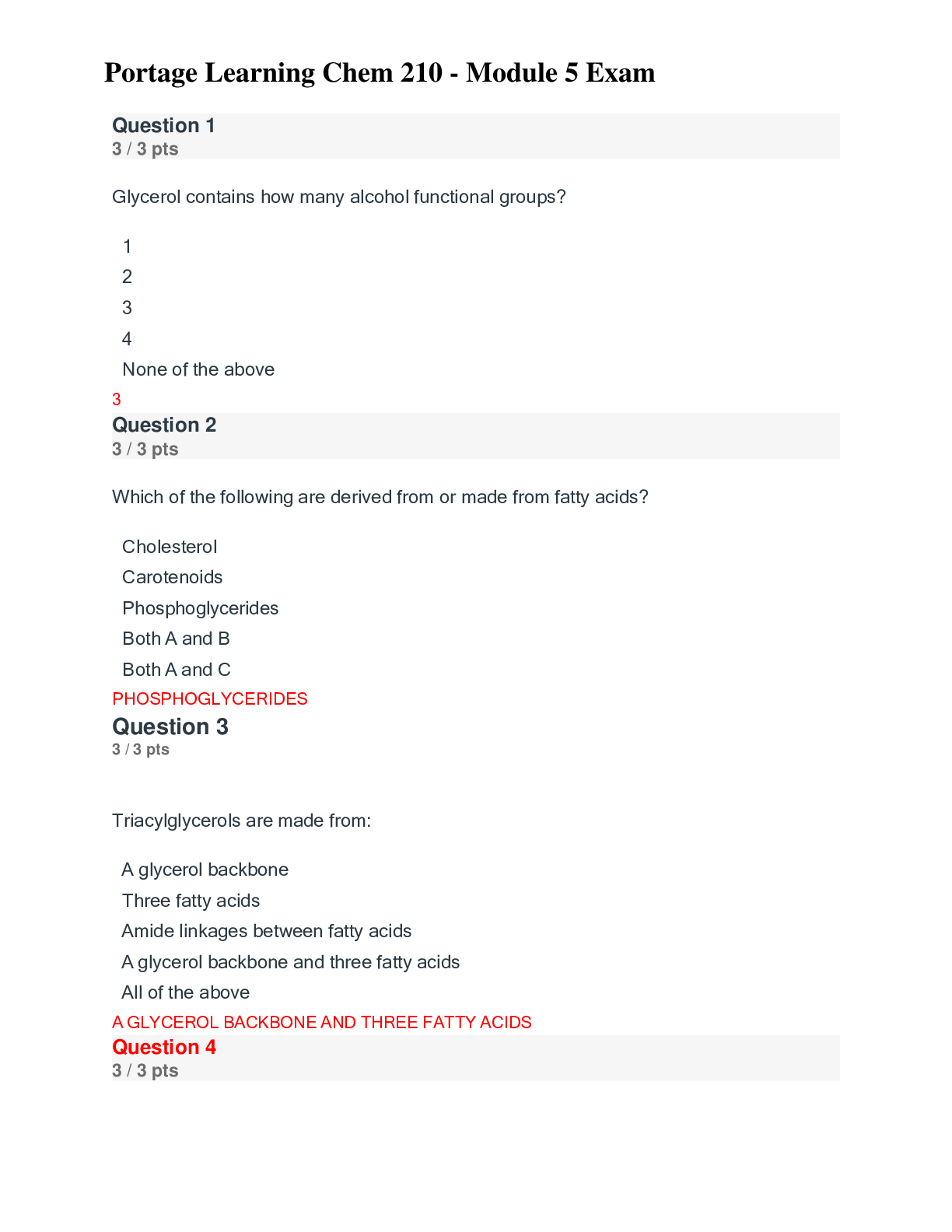
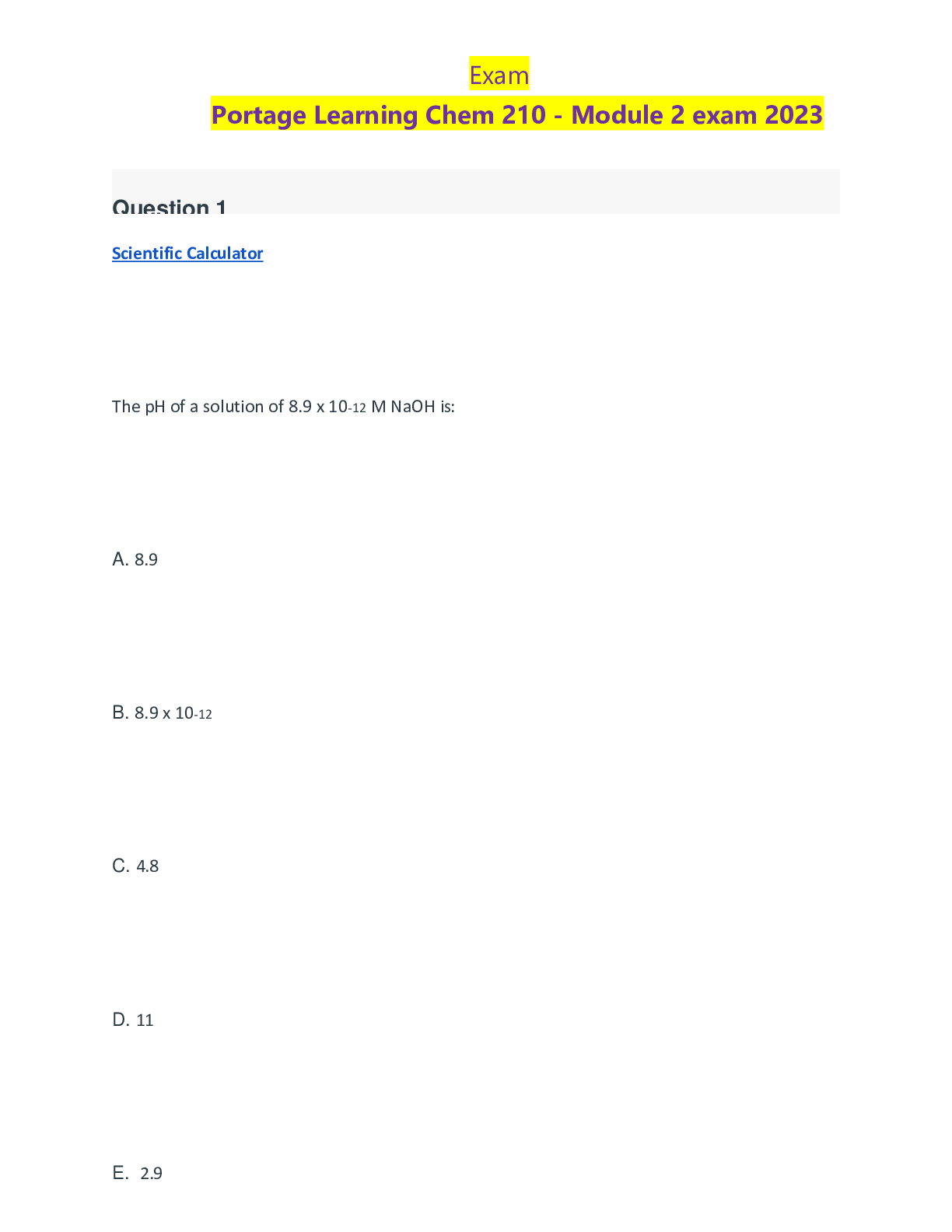
Question 1 3 / 3 pts Scientific Calculator The pH of a solution of 8.9 x 10-12 M NaOH is: A. 8.9 B. 8.9 x 10-12 C. 4.8 D. 11 E. 2.9 A B C D Correct! E [Kw=[H+][OH-] = 1.0 x 10-14; ... [H+][8.9 x 10-12] = 1.0 x 10-14 ;[H+]=890; pH=-log[890]=2.9] Question 2 3 / 3 pts Water is a unique molecule. Which of the following contributes to water’s unique place CHEM 210 MODULE 2 EXAM in the chemical world? The geometry of the molecule The polarity of the O-H bonds The ability of water molecules to hydrogen bond The bond angle of water All of the answers are correct ALL OF THE ANSWERS ARE CORRECT What factor contributes to the bent shape of a water molecule? The dipole arrows in a water molecule The unshared electron pairs on the oxygen atom The electronegativity difference between hydrogen and oxygen The unequal electron sharing between hydrogen and oxygen None of the answers are correct THE UNSHARED ELECTRON PAIRS ON TH [Show More]
Last updated: 1 year ago
Preview 1 out of 17 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 19, 2022
Number of pages
17
Written in
Additional information
This document has been written for:
Uploaded
May 19, 2022
Downloads
0
Views
52

 (2).png)


.png)




 (1).png)