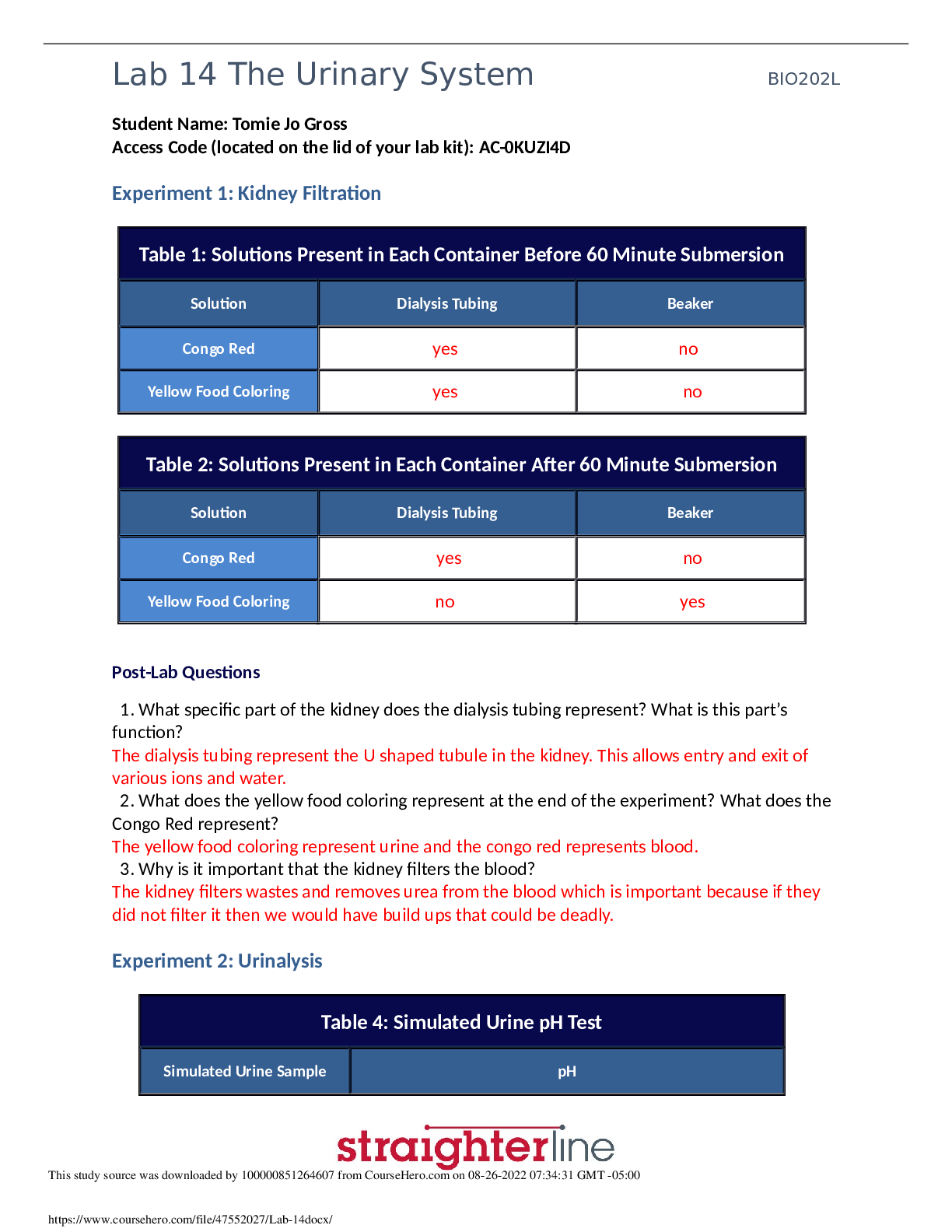
Physics > Lab Experiment > pH Scale Basics Remote Lab Albany State University PHYSICAL S 1011KpH Scale Basics Remote Lab1 (All)
pH Scale Basics Remote Lab Albany State University PHYSICAL S 1011KpH Scale Basics Remote Lab1
Document Content and Description Below
Learning Goals: Students will be able to: ● Determine if a solution is acidic or basic using pH. ● Relate liquid color to pH. ● Predict if dilution and volume will increase, decrease or not c... hange the pH ● Organize a list of liquids in terms of acid or base strength in relative order with supporting evidence. Everyday Chemistry in your life: 1. List some common liquids that you think are acidic or basic. For example, do you think orange juice is acid or base? Why do you think each liquid is an acid or base? a. orange juice is (Acid or base) and I think it is a common household item b. Water - is (Acid or base) and I think it is a acid/base because it is in the middle of the pH scale. c. Coffee is (Acid or base) and I think it is a common household item d. . Vomit is (Acid or base) and I think it has chemicals in it from all the food you eat. e. . Soap is (Acid or base) and I think has some chemicals but not enough to be an acid 2. Do you think the amount of the liquid changes how acidic or basic it is? Explain your thinking. No, because no matter the amount it still consists of the same ingredients whether it is a greater amount of liquid. 3. What do you think adding water (dilution) changes how acidic or basic the liquid is? Explain your thinking. I think that adding water to a acid or bases doesn’t make a difference because water has a pH of 7 which is neutral. Develop your understanding: 4. Use pH Scale Basics to test your ideas about some common things that are acids or bases. a. Describe your tests and results. b. Were all of the liquids you listed in #1 in the simulation? If not, research to find the pH and identify each as acid or base. If all your items were in the simulation, look up a few other common things. (cite references) c. OJ- is a ACID AND HAS A pH 3.50. Water-is a NEUTRAL AND HAS A pH of 7.00 Coffee- is ACID AND HAS A pH of 5.00 Vomit- is a ACID AND HAS A pH of 2.00 Soap- is BASE AND HAS A pH of 10.00 4. Experiment to check your ideas from #2 about how color or volume help identify whether something is acid, base, or neutral. Describe your tests and results with specific examples. Color effects the identity of water because of the certain pH level. For Soap when I put 1 liter into the container the level of pH didn’t change neither did scale ranking. 5. Experiment to check your ideas from #3 about whether dilution will increase, decrease or not change the pH. a. Does every solution behave the same way? For acids when you add water the level of pH increases but for Bases when you put water the level of pH decreases. b. Explain how you can use pH to help predict what dilution does to an acid or base solution. You can use pH to help predict what dilution does to acid or base by looking at he level of pH it is on. 6. Consider some common drinks Alkaline water 8.0, Fruit juice 6.8, Gatorade 3.0, Green tea 9.0, Gatorade 3.0, Vinegar 3.5, a. Organize this list of foods with pH from most basic to most acidic. Gatorade, Vinegar, Fru [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 15, 2021
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
May 15, 2021
Downloads
0
Views
52

.png)


.png)
















.png)