Chemistry > QUESTIONS & ANSWERS > North Carolina State University CH CH101 CHEMISTRY 101 Lab 10 InLab - Acid-Base Studies. Curren (All)
North Carolina State University CH CH101 CHEMISTRY 101 Lab 10 InLab - Acid-Base Studies. Current Score : 50 / 50
Document Content and Description Below
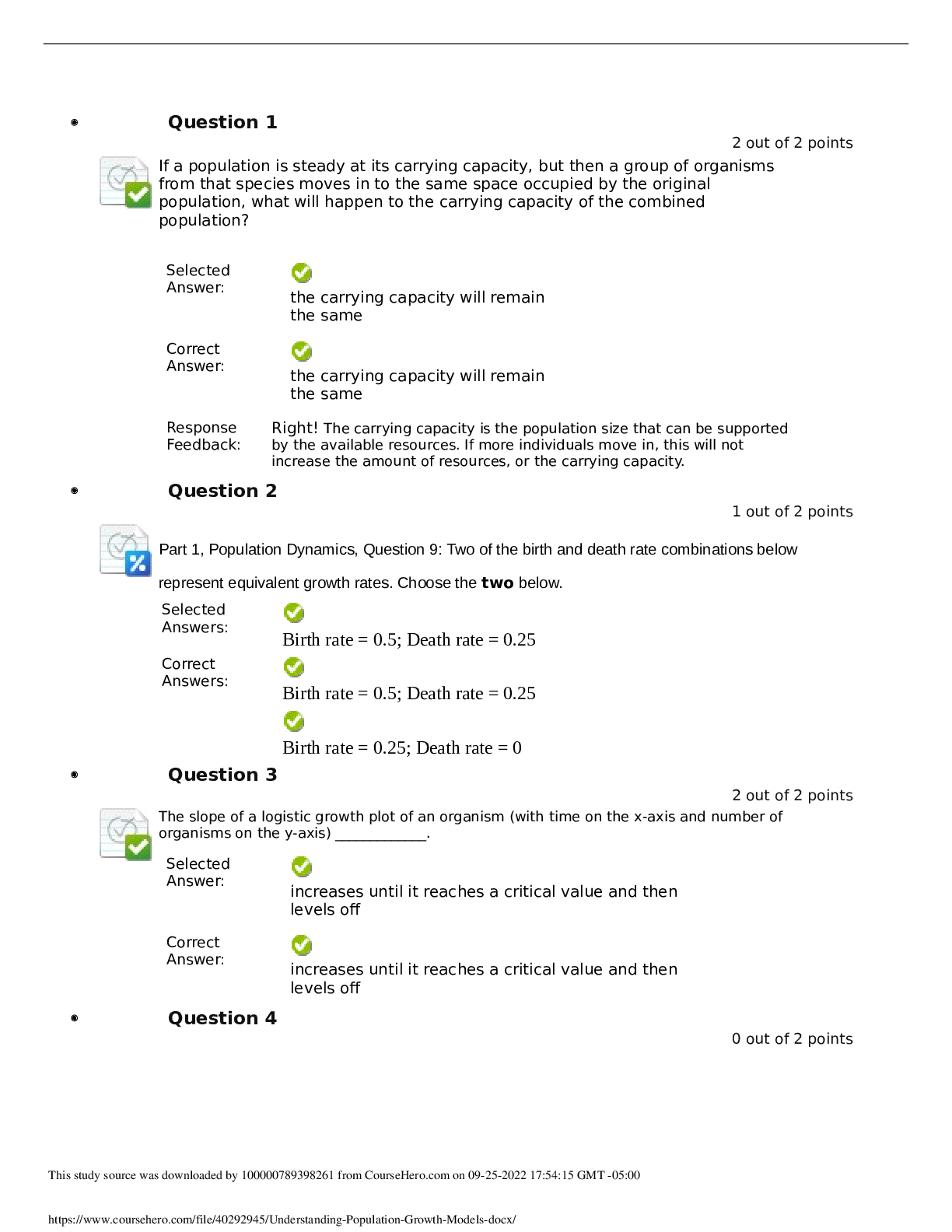
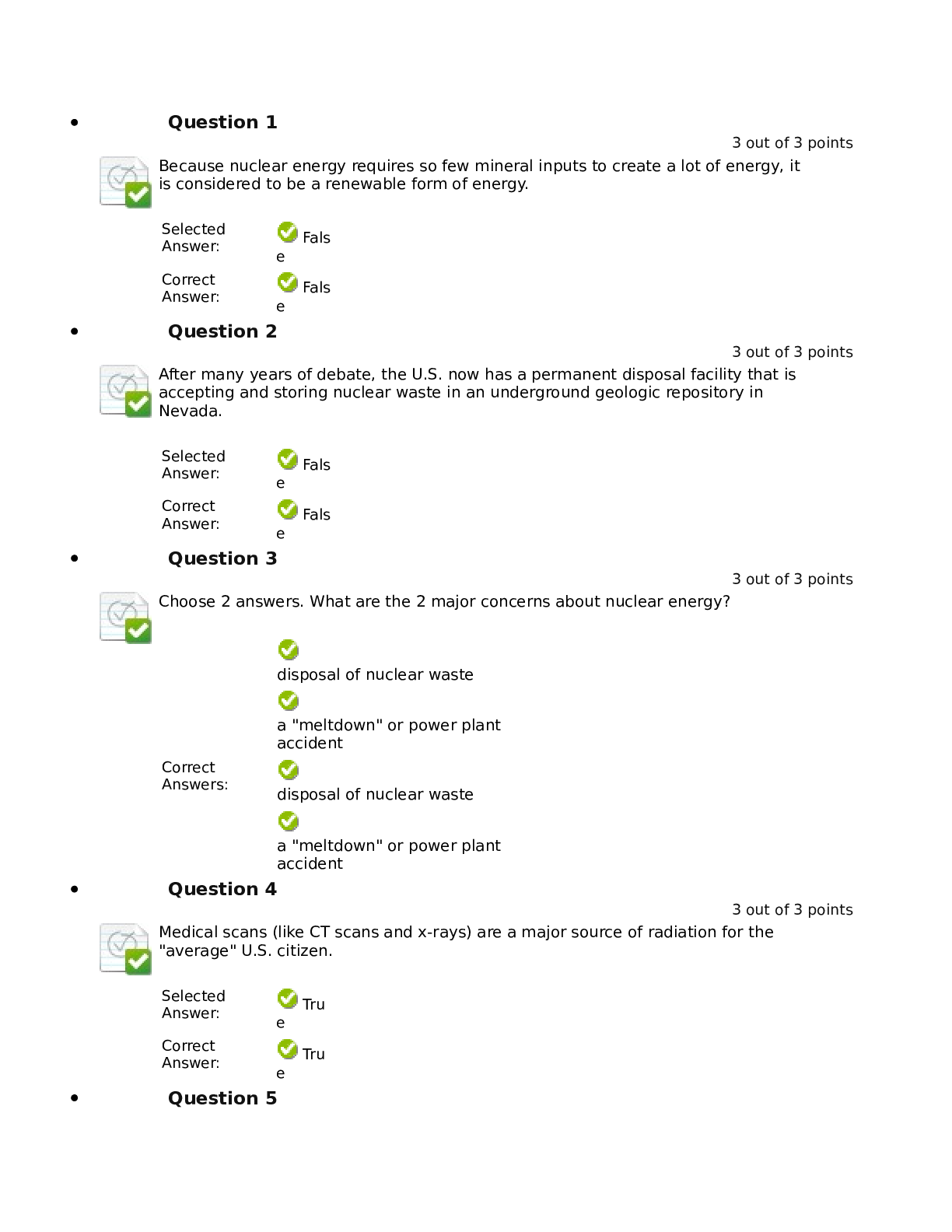
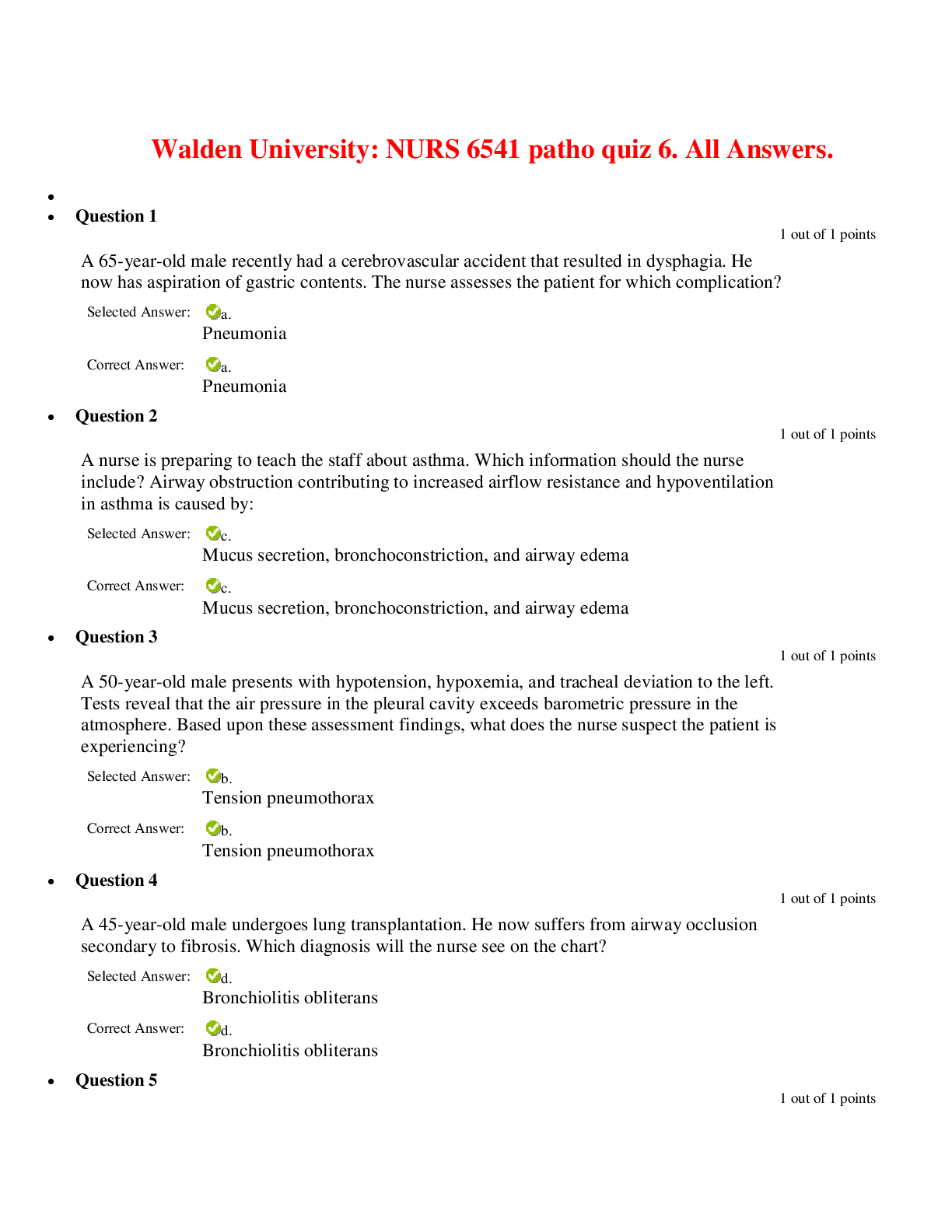
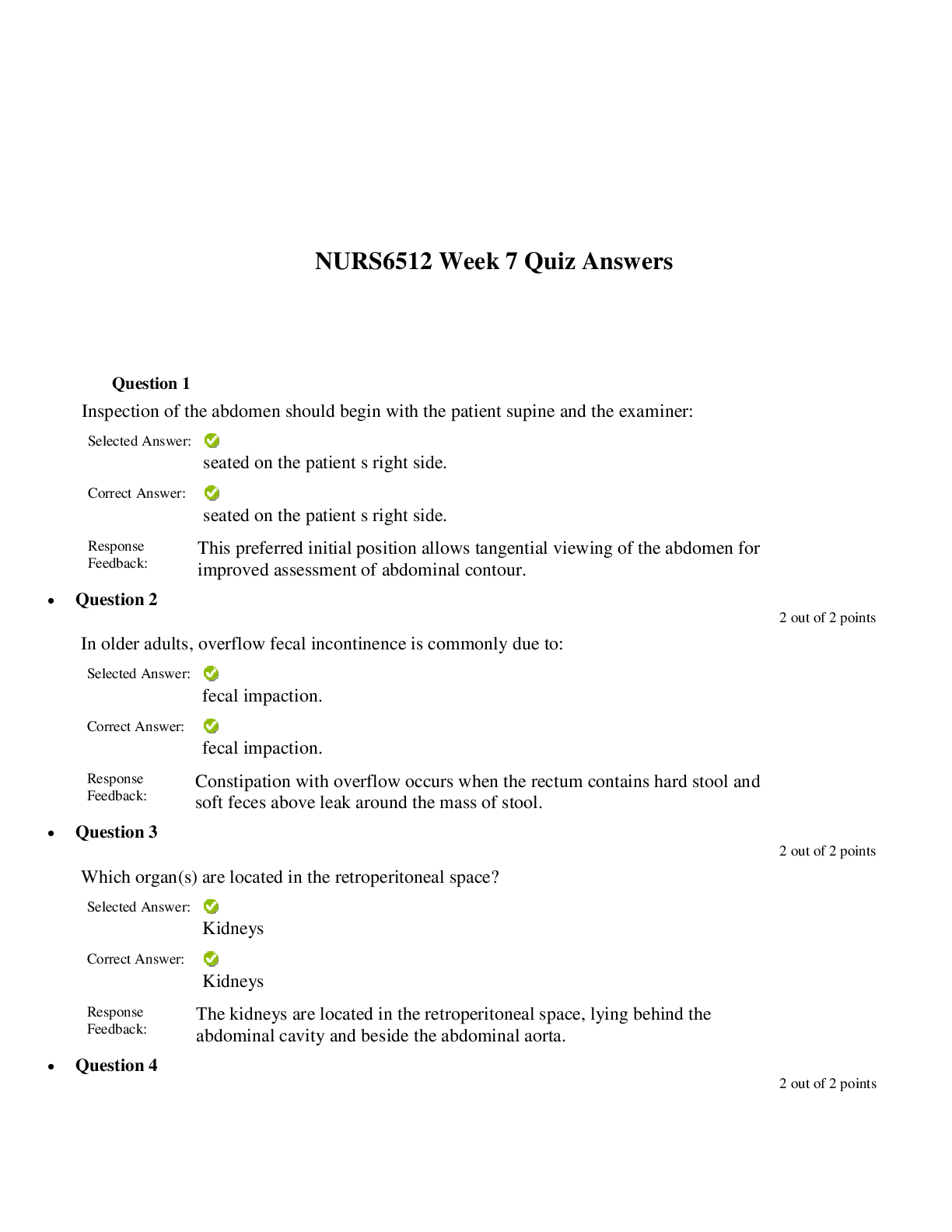
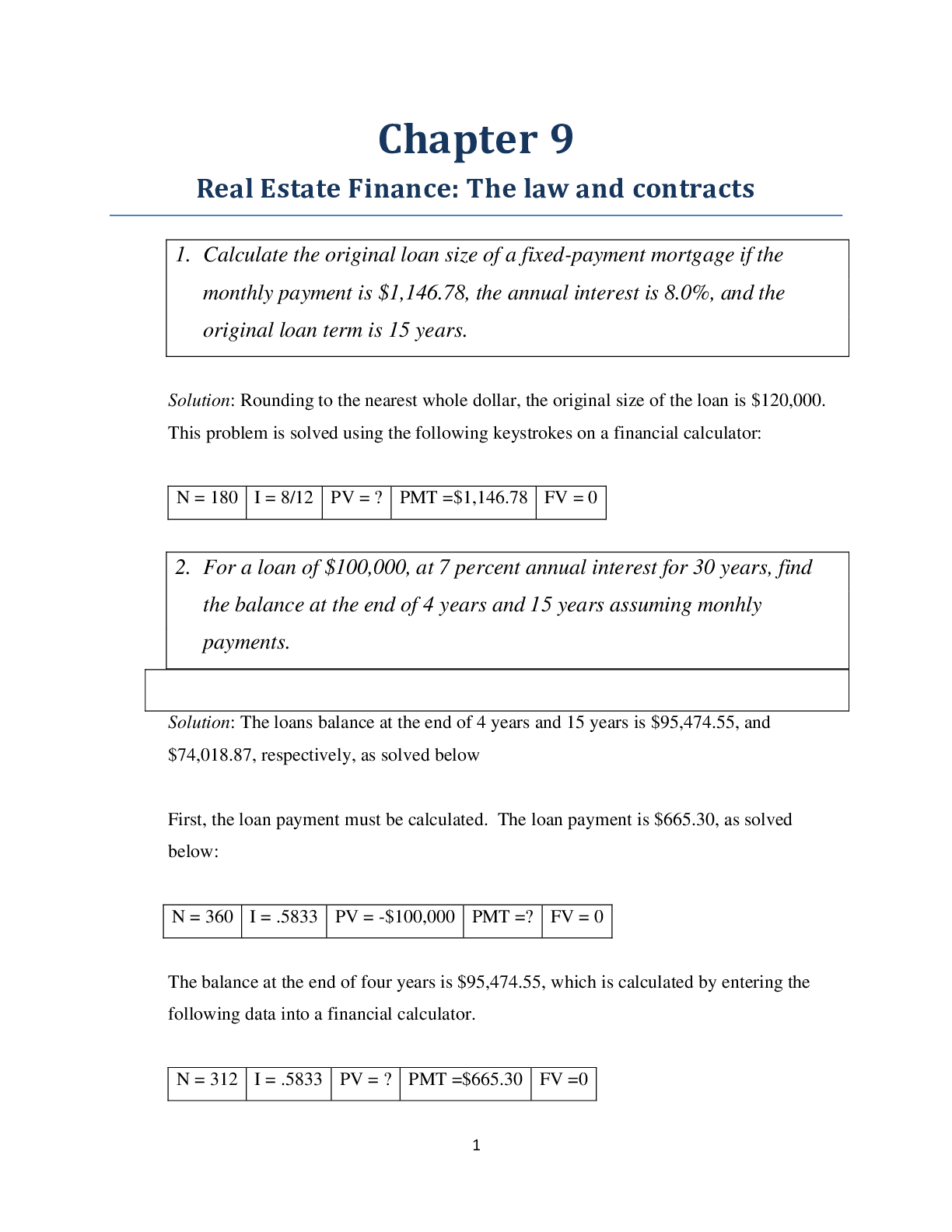
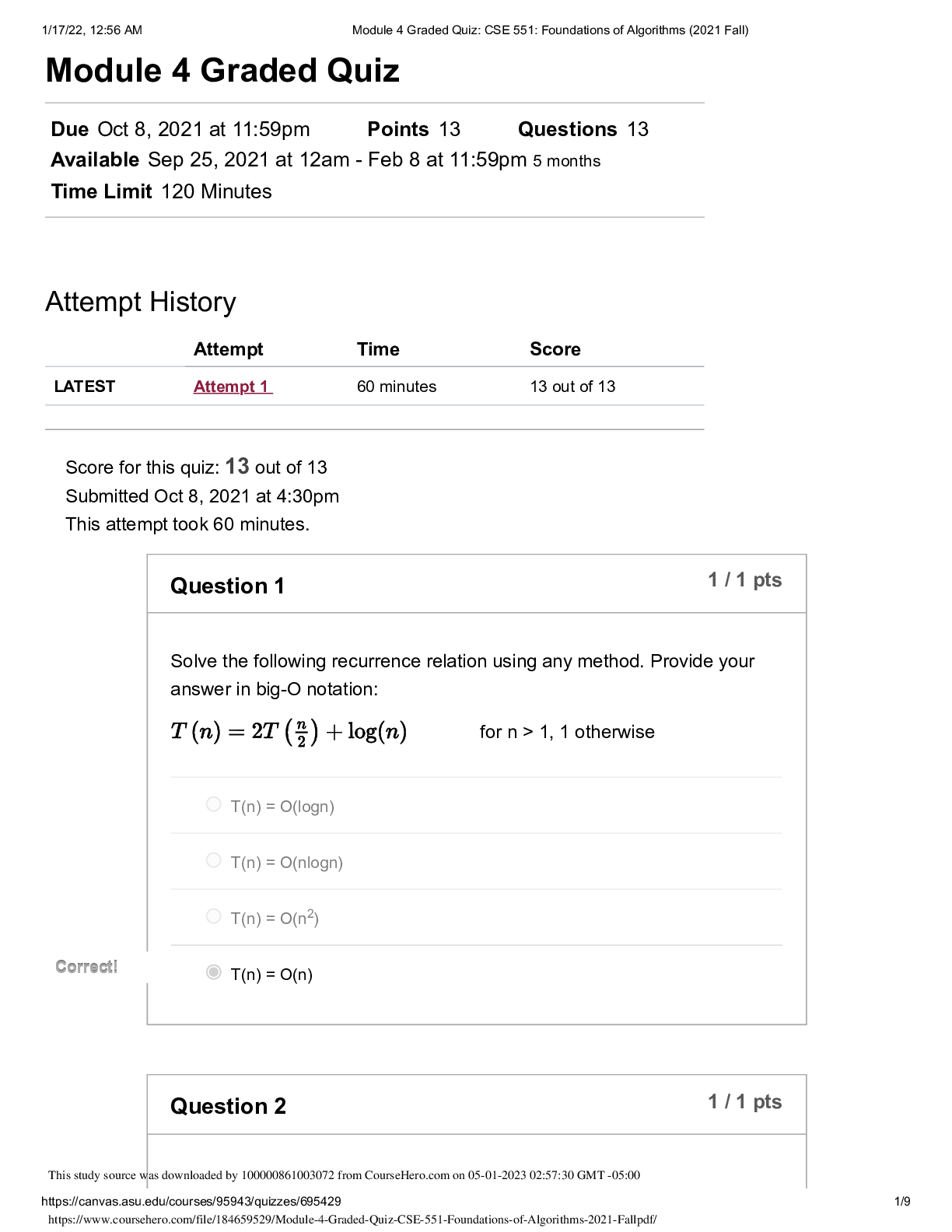
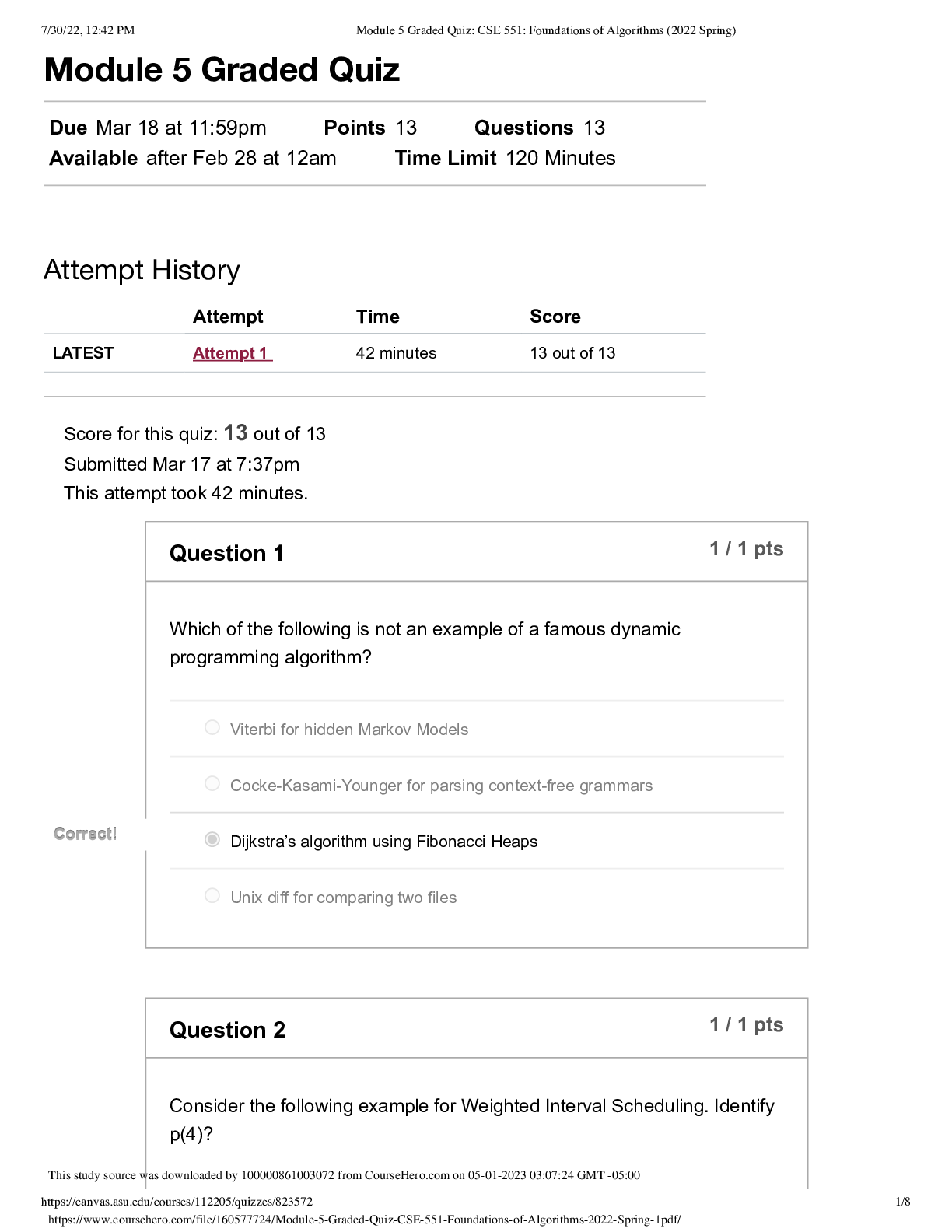
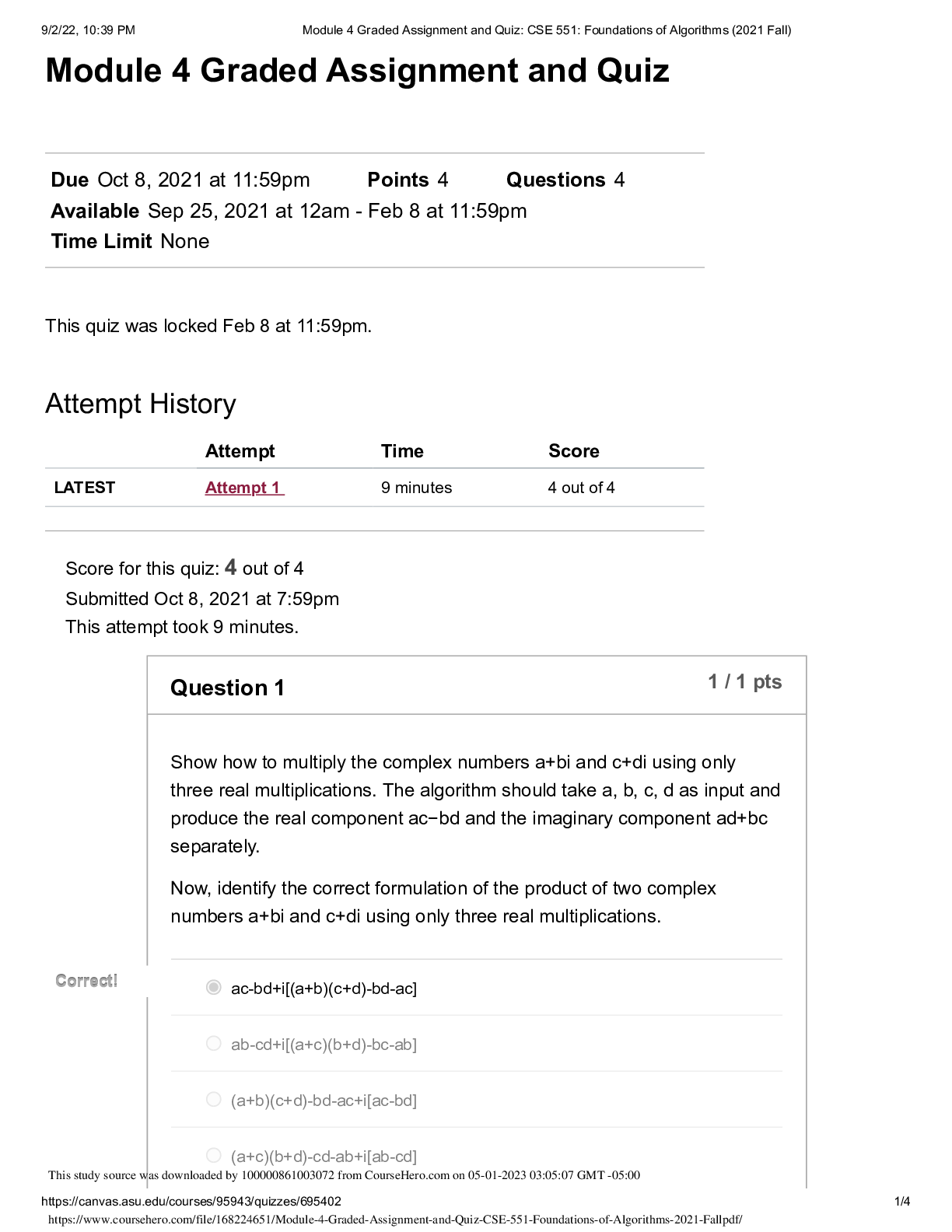
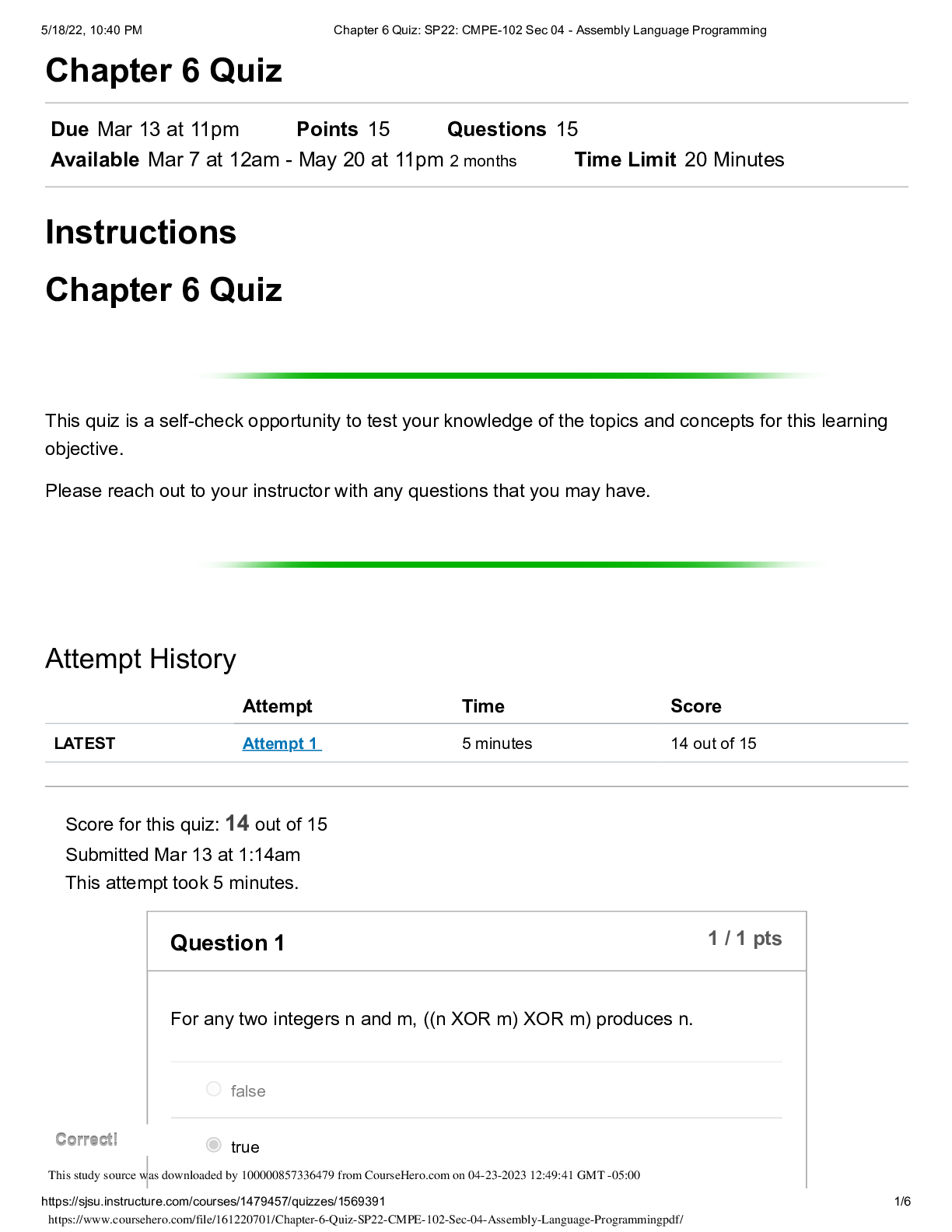
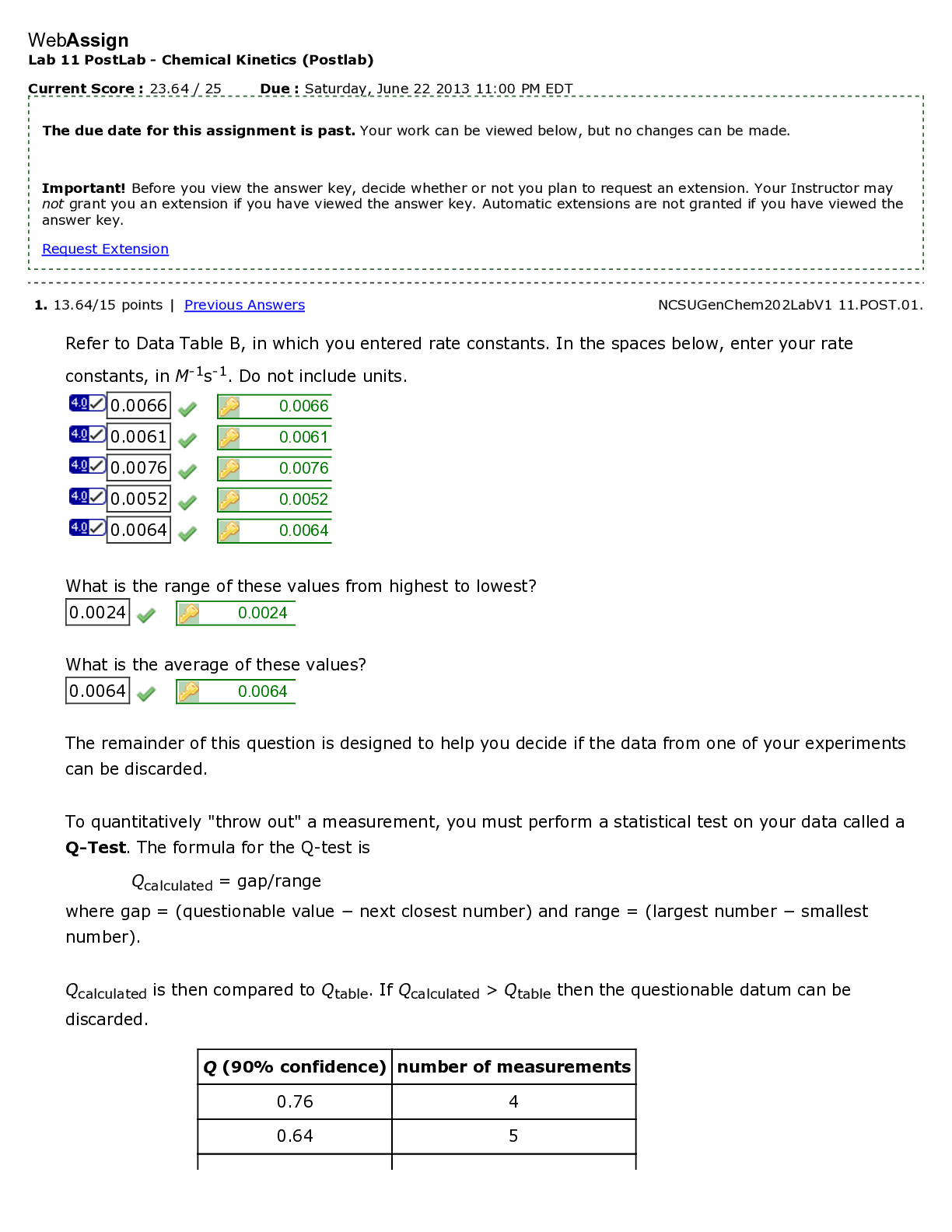
North Carolina State University CH CH101 CHEMISTRY 101 Lab 10 InLab - Acid-Base Studies Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-R... esponses/last?dep=10120925 Page 1 of 9 Current Score : 50 / 50 Due : Friday, November 14 2014 01:10 PM EST 1. 1.2/1.2 points | Previous Answers Performing a high quality calibration of a pH probe can be challenging. Once the pH probe calibration is complete, it is a good idea to test the pH of each of the buffer solutions used to calibrate the probe. This will provide information on how accurate the calibration is prior to reading experimental values. If the measured pH of a buffer solution is more than 1.00 pH unit different than the given value of the solution, consider replacing that buffer solution and calibrating again. Record your readings below as a record of how accurate your pH calibration is. Calibration of pH probe Red Buffer Solution pH = 4.00 Yellow Buffer Solution pH = 7.00 Blue Buffer Solution pH = 10.00 4.01 6.82 10.12 2. 7.5/7.5 points | Previous AnswersNCSUGenChem102LabV1 10.IL.01. Part A: pH Measurements of Some Common Acid and Base Solutions Complete the following table. (Enter your pH values to the 0.1 place.) Data Table A: pH measurements of acids and bases commonly found in a chemistry laboratory. number solution pH 1 0.010 M HCl 1.8 2 0.0010 M HCl 2.7 3 0.00010 M HCl 3.6 4 0.010 M HC2H3O2 2.9 5 0.010 M NaOH 11.1 Lab 10 InLab - Acid-Base Studies (In-Lab) Benton Gorre CH 102, section 111, Fall 2014 Instructor: Pallavi Singh TA WebAssign The due date for this assignment is past. Your work can be viewed below, but no changes can be made. Important! Before you view the answer key, decide whether or not you plan to request an extension. Your Instructor may not grant you an extension if you have viewed the answer key. Automatic extensions are not granted if you have viewed the answer key. Request Extension View Key Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 2 of 9 6 0.0010 M NaOH 10.1 7 0.010 M NH3 10.1 Recall from your lab manual that [H3O+ ] = 10-pH and that Kw = [H3O+ ] · [OH- ] = 1.0 ✕✕ 10-14. Using your measured pH values, calculate the [H3O+ ] and [OH- ] for solutions 1, 4, 5 and 7. (Enter all calculations to two significant figures. Very small and very large values should be entered using scientific notation. For example, 0.000163 should be entered as 1.6e-4.) number solution [H 3O+] [OH- ] 1 0.010 M HCl 1.6e-2 6.3e-13 4 0.010 M HC2H3O2 1.3e-3 7.9e-12 5 0.010 M NaOH 7.9e-12 1.3e-3 7 0.010 M NH3 7.9e-11 1.3e-4 Additional Materials Acid-Base Studies Lab Safety and Practices Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 3 of 9 3. 4.8/4.8 points | Previous AnswersNCSUGenChem102LabV1 10.IL.02. Green checks and red X's are not displayed for this question. In-Lab Question 1. Comparing the concentration of your solutions with the hydronium or hydroxide concentration, select the response that is suggested by your pH measurements. (a) For solution 1, the [HCl] is approximately equal to [H3O+ ], suggesting that HCl is a strong acid and that K >> 1 . (b) For solution 4, the [HC2H3O2] is much greater than [H3O+ ], suggesting that HC2H3O2 is a weak acid and that K << 1 . (c) For solution 5, the [NaOH] is approximately equal to [OH- ], suggesting that NaOH is a strong base and that K >> 1 . (d) For solution 7, the [NH3] is much greater than [OH- ], suggesting that NH3 is a weak base and that K << 1 . Additional Materials Acid-Base Studies Lab Safety and Practices 4. 1.5/1.5 points | Previous AnswersNCSUGenChem102LabV1 10.IL.03. Green checks and red X's are not displayed for this question. In-Lab Question 2a. What happened to the pH when the 0.010 M HCl was diluted to 0.0010 M HCl? In-Lab Question 2b. What happened to the pH when the 0.010 M NaOH was diluted to 0.0010 M NaOH? It increased. It decreased. It did not change. Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 4 of 9 In-Lab Question 2c. State a general rule about what happens to the pH of acidic or basic solutions when they are diluted with pure water. Additional Materials Acid-Base Studies Lab Safety and Practices It increased. It decreased. It did not change. Diluting a strong acid results in a lower concentration of hydronium, which decreases the pH. Diluting a strong base results in a lower concentration of hydroxide, which increases the pH. Diluting a strong acid or base will always result in a decrease of the pH. Diluting a strong acid results in a lower concentration of hydronium, which increases the pH. Diluting a strong base results in a lower concentration of hydroxide, which decreases the pH. Diluting a strong acid or base will always result in an increase of the pH. Diluting a strong acid or base does not change the pH at all because these materials dissociate completely. Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 5 of 9 5. 9/9 points | Previous AnswersNCSUGenChem102LabV1 10.IL.04. Green checks and red X's are not displayed for this question. Part B: Acidity and Basicity of Some Household Chemicals Complete the following table. (Enter your pH values to the nearest whole number.) Data Table B: Acidity and basicity of some household chemicals substance pH acid/base/neutral vinegar 2 acidic bleach 8 basic vitamin C 3 acidic lemon juice 2 acidic baking soda 11 basic dishwasher detergent 9 basic carbonated water 6 acidic baking powder 11 basic ammonia 12 basic Additional Materials Acid-Base Studies Lab Safety and Practices 6. 1.5/1.5 points | Previous AnswersNCSUGenChem102LabV1 10.IL.05. In-Lab Question 3a. Select all of the household chemicals that you found to be acidic. (Select all that apply.) ammonia baking powder baking soda bleach carbonated water dishwasher detergent lemon juice vinegar vitamin C none Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 6 of 9 In-Lab Question 3b. Select all of the household chemicals that you found to be basic. (Select all that apply.) ammonia baking powder baking soda bleach carbonated water dishwasher detergent lemon juice vinegar vitamin C none In-Lab Question 3c. Select all of the household chemicals that you found to be neutral. (Select all that apply.) ammonia baking powder baking soda bleach carbonated water dishwasher detergent lemon juice vinegar vitamin C none Additional Materials Acid-Base Studies Lab Safety and Practices 7. 4/4 points | Previous AnswersNCSUGenChem102LabV1 10.IL.06. For Data Table B, the pH of baking soda (sodium bicarbonate) was measured. Answer the following questions based on your observations. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) (a) Write the dissolution reaction for solid sodium bicarbonate below. NaHCO3(s) → Na1+ (aq) + HCO 1- 3 Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 7 of 9 (aq) NaHCO_3(s) --> Na^1+ (aq) + HCO_3^-1 (aq) Once the ionic solid has dissolved, the amphiprotic anion that is formed is able to react as an acid or as a base with water. (For parts b and c, omit states-of-matter in your answer.) (b) Write the acid-base reaction where hydrogen carbonate is the acid and water is the base. HCO 1- 3 + H2O → CO 2- 3 + H3O1+ HCO_3^1- + H_2O --> CO_3^2- + H_3O^1+ (c) Write the acid-base reaction where water is the acid and hydrogen carbonate is the base. H2O + HCO 1- 3 → OH1- + H2CO3 H_2O + HCO_3^1- --> OH^-1 + H_2CO_3 (d) Based upon the pH you measured, which statement is accurate? The reaction of hydrogen carbonate as an acid with water is more extensive because the measured pH is less than 7. The reaction of hydrogen carbonate as a base with water is more extensive because the measured pH is greater than 7. The reaction of hydrogen carbonate as an acid with water is more extensive because the measured pH is greater than 7. The reaction of hydrogen carbonate as a base with water is more extensive because the measured pH is less than 7. Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 8 of 9 Additional Materials Acid-Base Studies Lab Safety and Practices 8. 5.5/5.5 points | Previous AnswersNCSUGenChem102LabV1 10.IL.07. Part C: Acid-Base Reactions Write the acid-base reaction that occurs when an aqueous solution of HCl is added to an aqueous solution of NaOH. (Use the lowest possible coefficients. Omit states-of-matter in your answer.) H3O1+ + OH1- → 2H2O H_3O^1+ + OH^1- --> 2H_2O Complete the following table. (Enter your pH values to the 0.1 place.) Green checks and red X's are not displayed for the classification portion of this question. Data Table C: Reaction of HCl and NaOH mL NaOH pH classification 0.0 1.6 acidic 3.0 1.8 acidic 6.0 2.4 acidic 12.0 11.1 basic Additional Materials Acid-Base Studies Lab Safety and Practices Lab 10 InLab - Acid-Base Studies 11/25/14, 12:43 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120925 Page 9 of 9 9. 15/15 points | Previous AnswersNCSUGenChem102LabV1 1.IL.04. Type your name into the answer box. This space will be used by your instructor to record your points for class participation in the lab using the Lab Participation Rubric. Benton Gorre Score: 15 out of 15 Comment: Additional Materials Lab Safety and Practices [Show More]
Last updated: 1 year ago
Preview 1 out of 9 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 06, 2022
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Oct 06, 2022
Downloads
0
Views
54








.png)





IIUUKK.png)
.png)
.png)
.png)

.png)
 (1)ACADC.png)
 (1)ADQEFQEFEQ.png)
 (1)CDWDCW.png)