Chemistry > QUESTIONS & ANSWERS > North Carolina State University CH CH101 CHEMISTRY 101 Lab 9 PreLab - Titrations. Current Score : (All)
North Carolina State University CH CH101 CHEMISTRY 101 Lab 9 PreLab - Titrations. Current Score : 25 / 25 Due
Document Content and Description Below
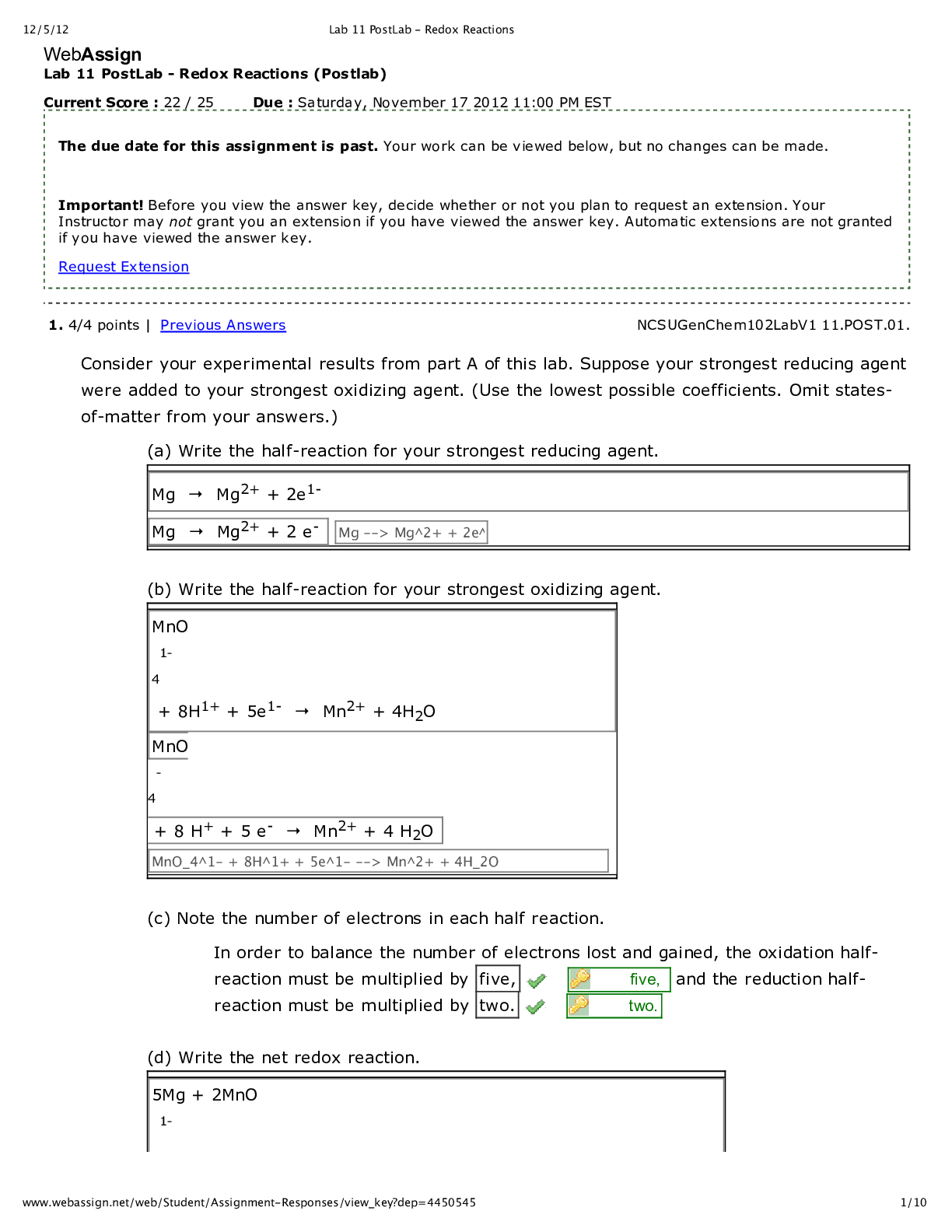
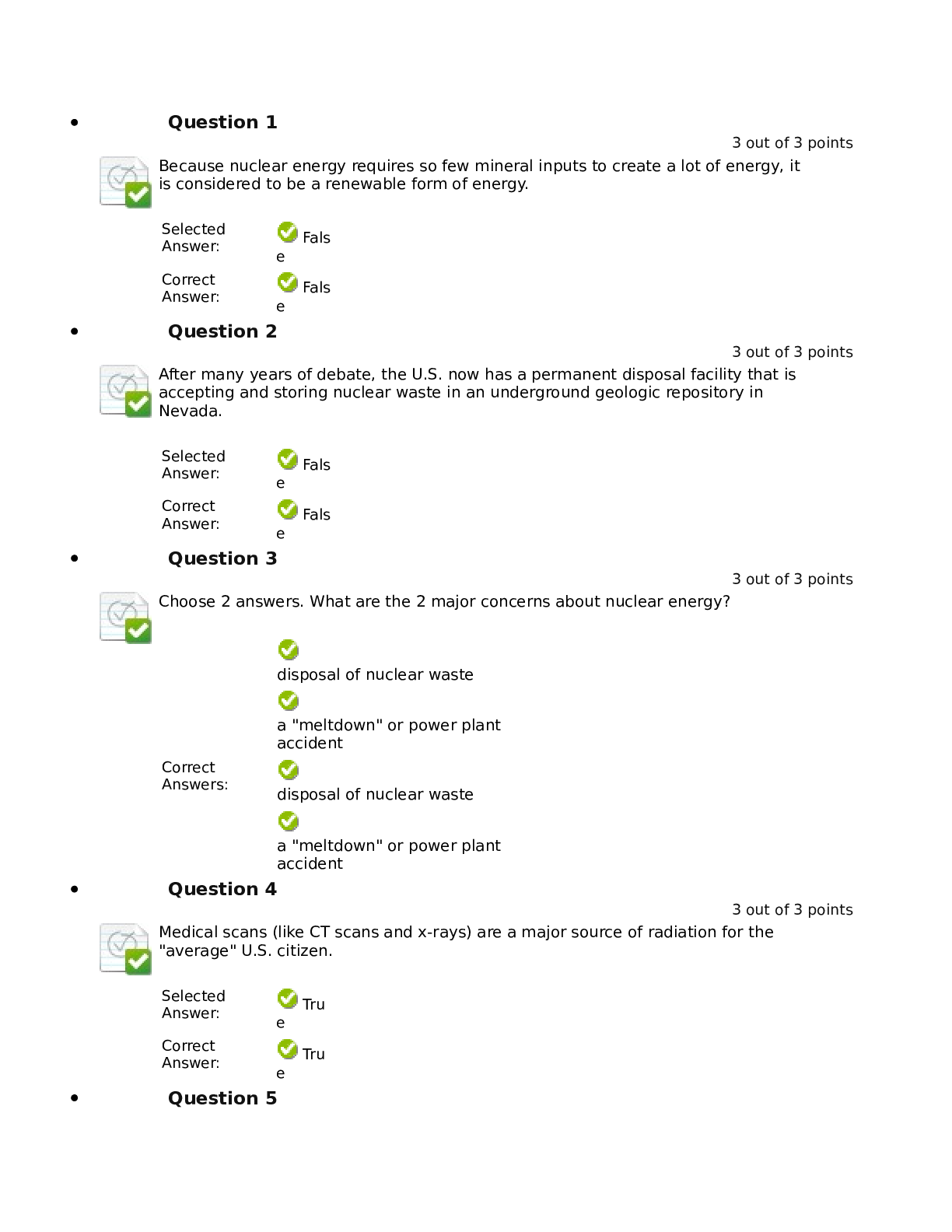
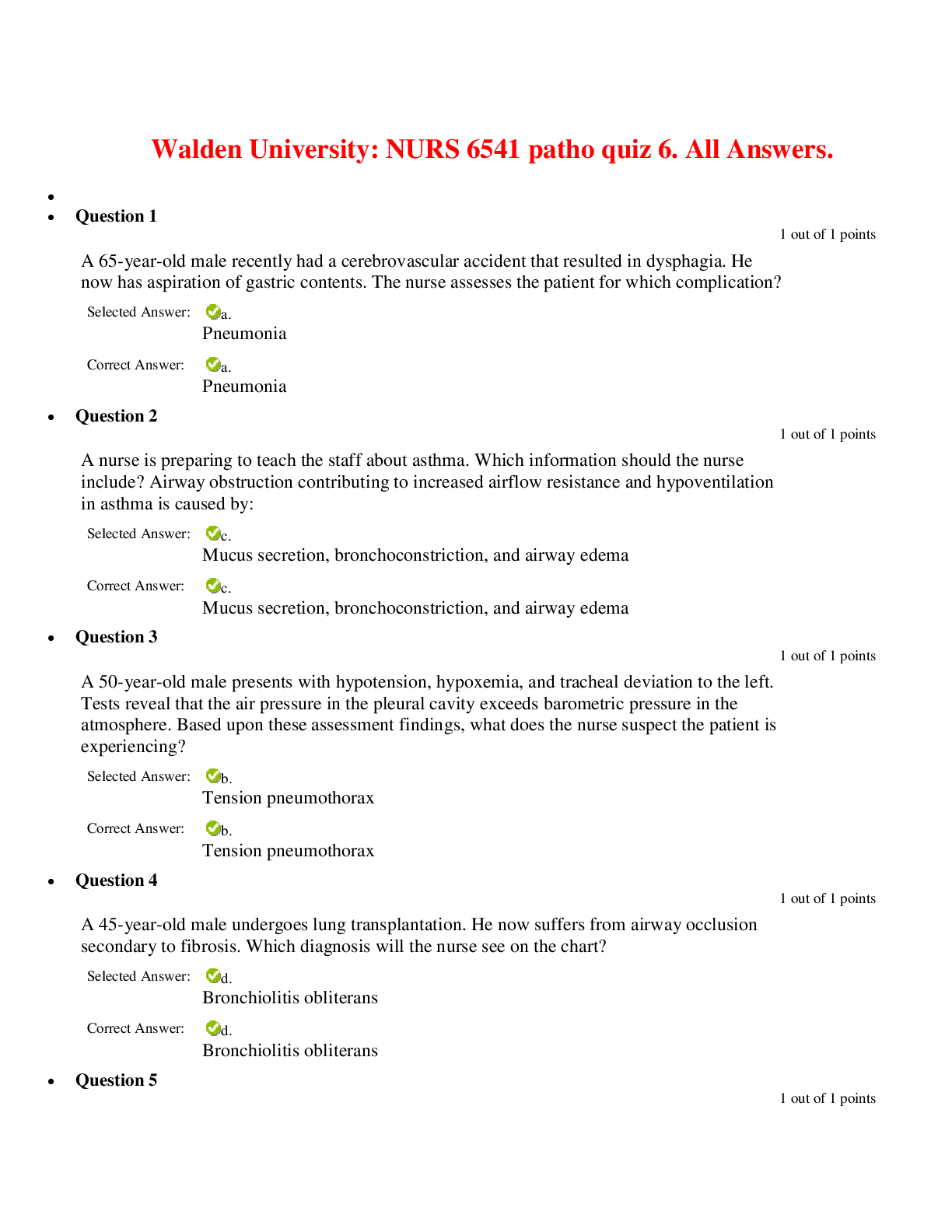
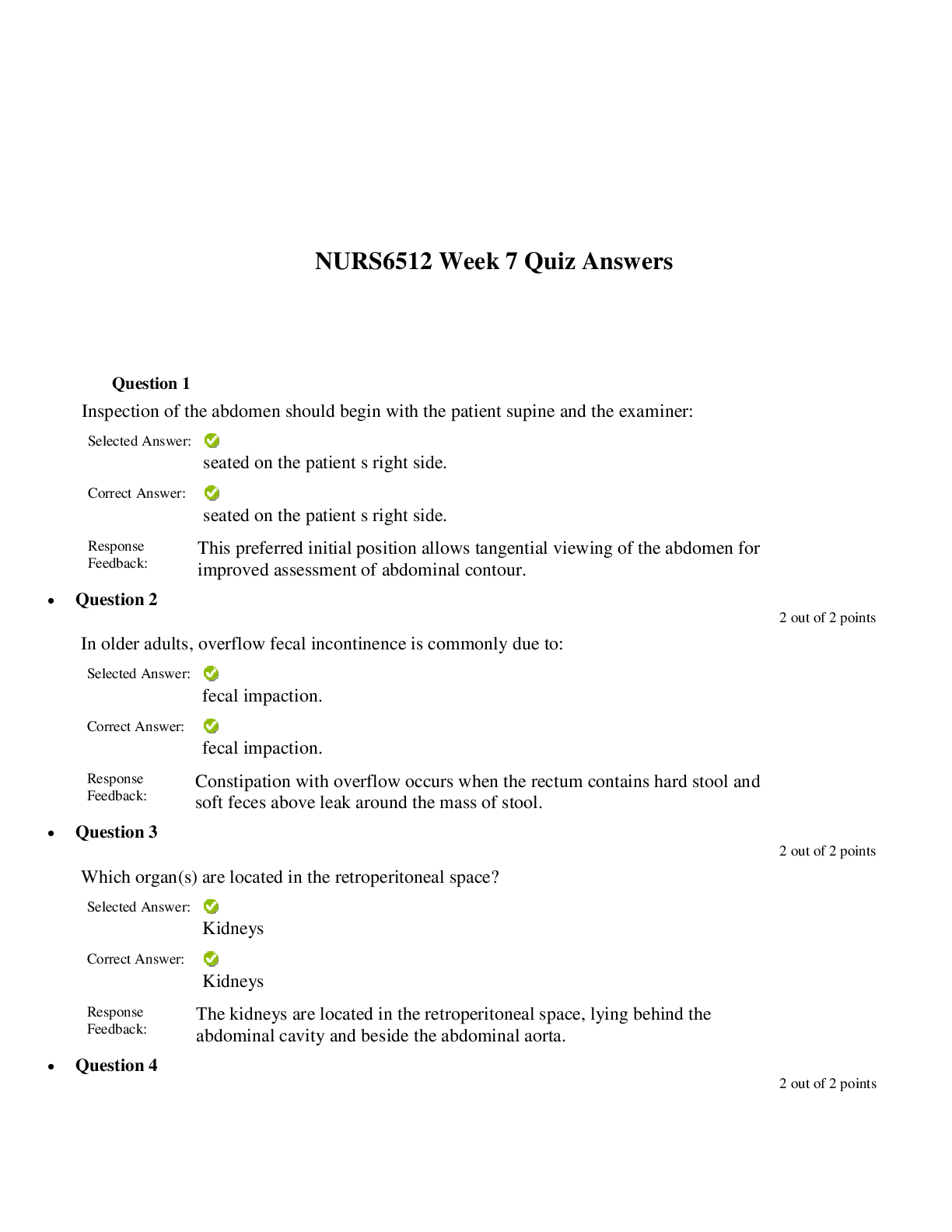
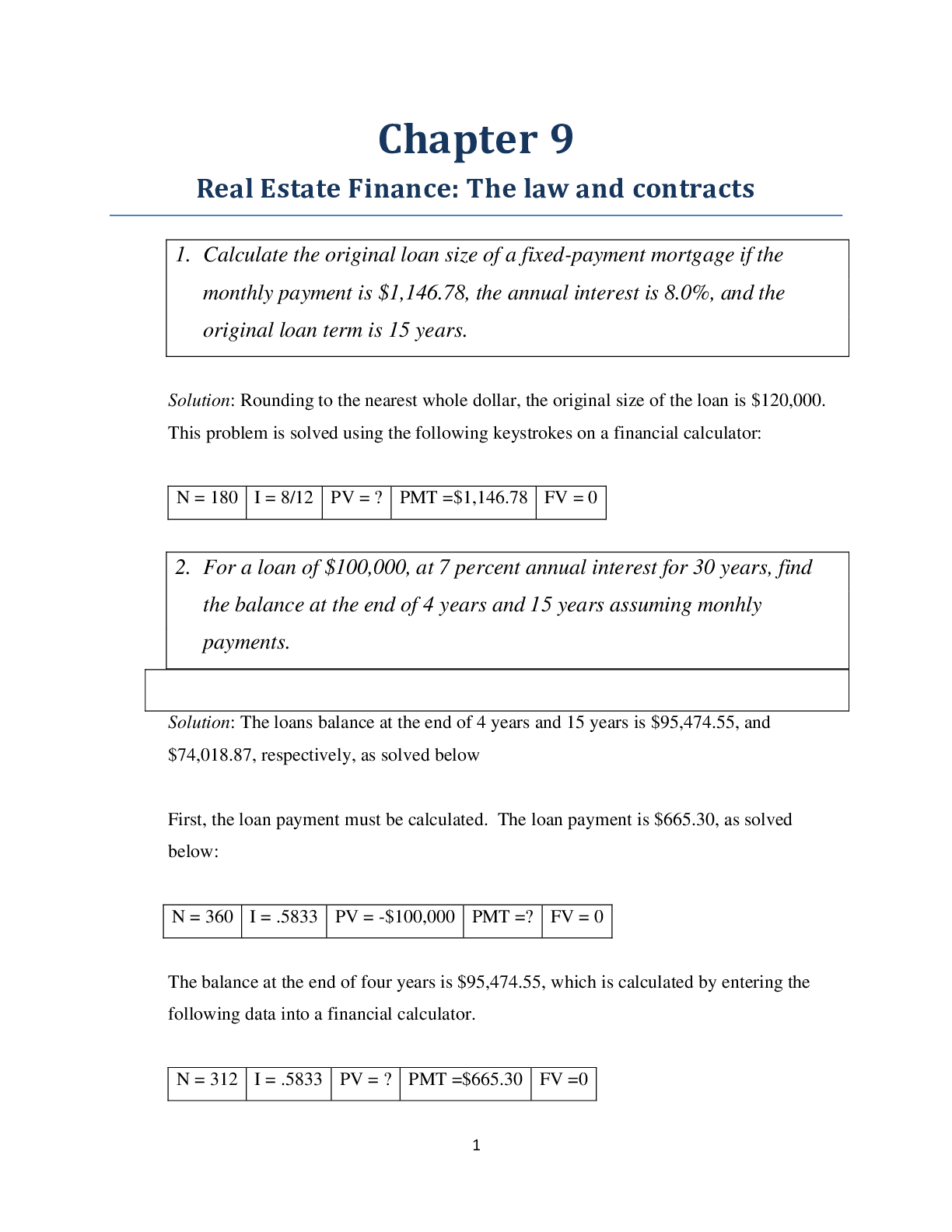
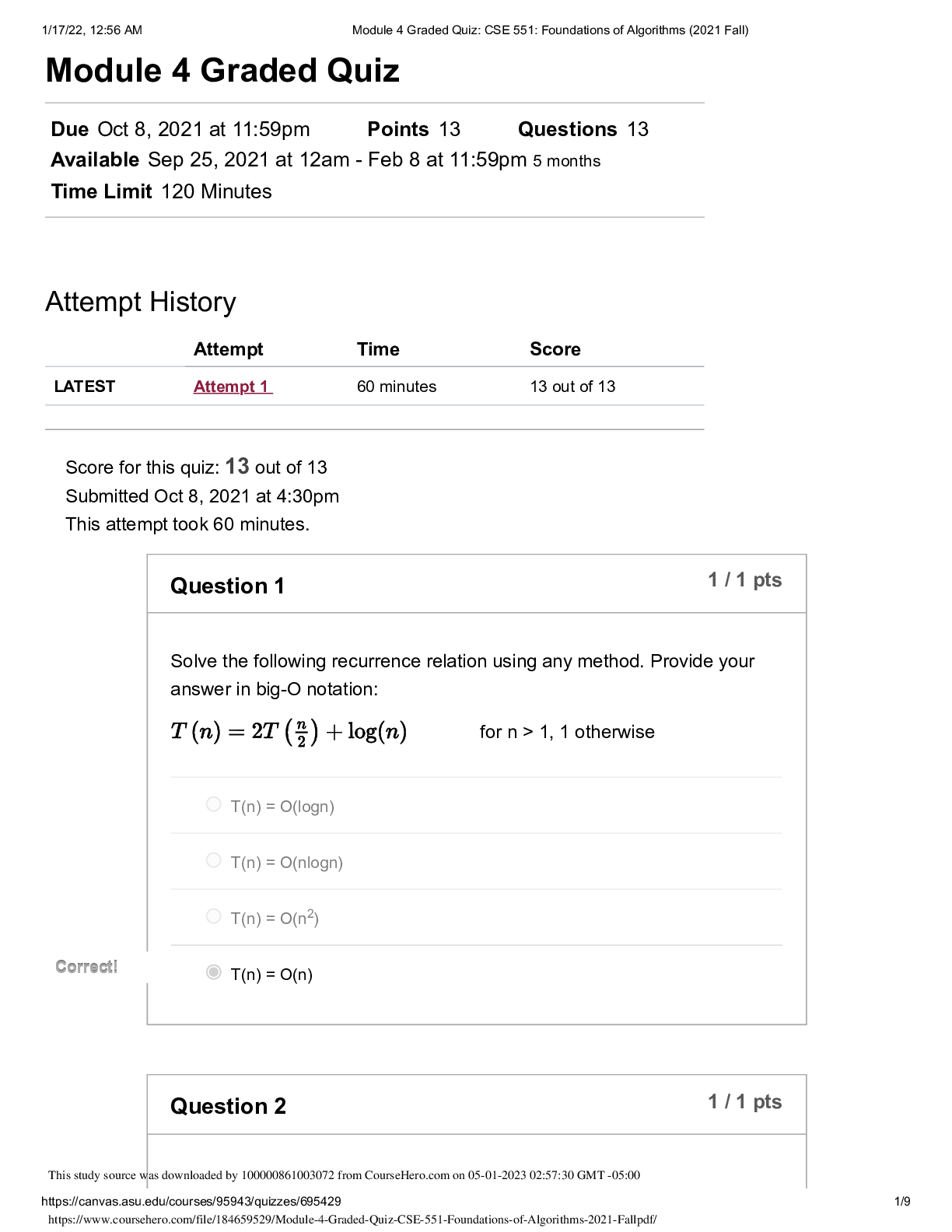
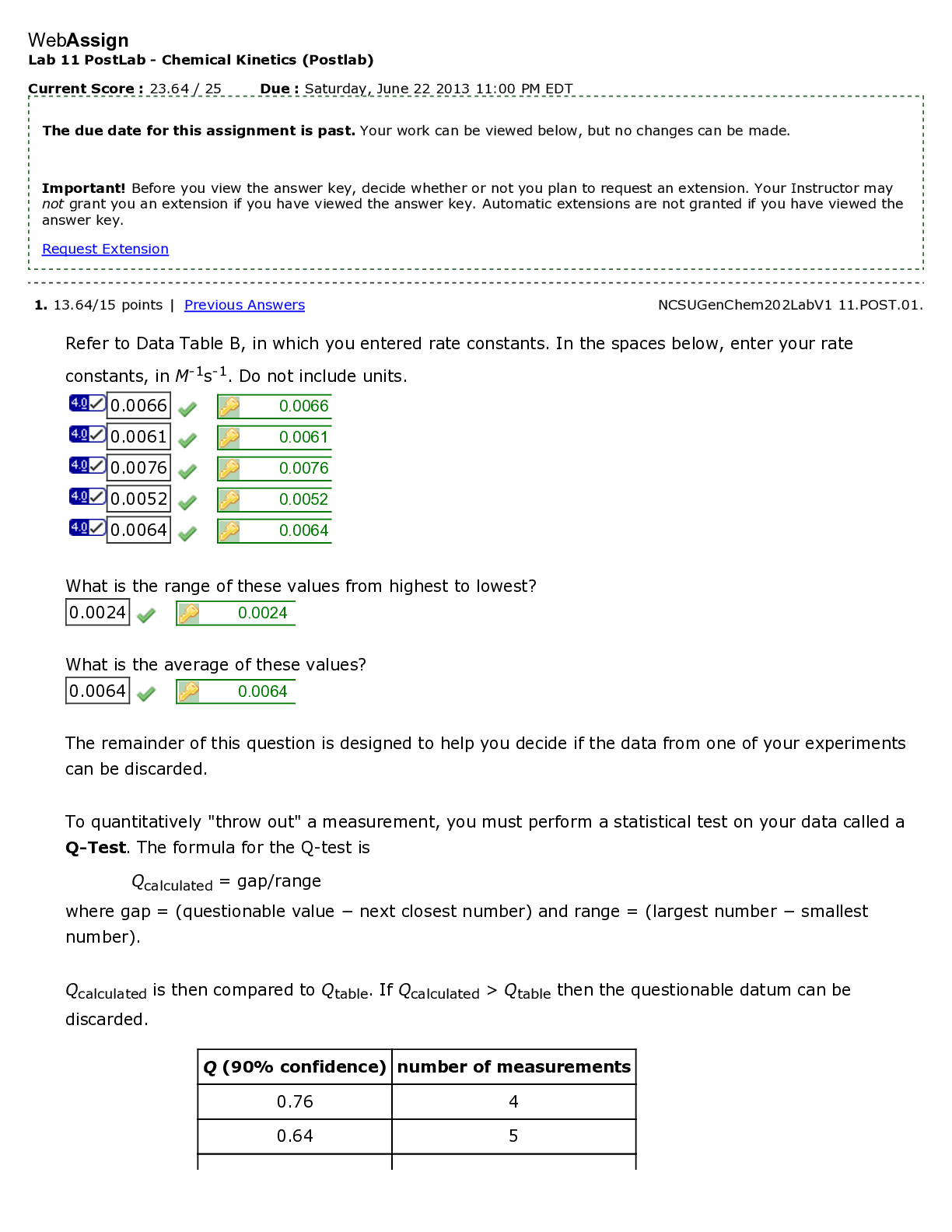
Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 1 of 7 Current Score : 25 / 25 Due : Thursday, November 6 2014 11:00 PM ... EST 1. 5/5 points | Previous AnswersNCSUGenChem102LabV1 9.PRE.01. Green checks and red X's are not displayed for this question. The following items address safety issues in the Titrations Lab. (Select all that apply.) (a) Sodium hydroxide (NaOH) is listed as which of the following? (b) Vinegar is listed as which of the following? (c) Phenolphthalein is listed as which of the following? Lab 9 PreLab - Titrations (Prelab) Benton Gorre CH 102, section 111, Fall 2014 Instructor: Pallavi Singh TA WebAssign The due date for this assignment is past. Your work can be viewed below, but no changes can be made. Important! Before you view the answer key, decide whether or not you plan to request an extension. Your Instructor may not grant you an extension if you have viewed the answer key. Automatic extensions are not granted if you have viewed the answer key. Request Extension View Key corrosive irritating vapors toxic if ingested causes staining on skin flammable none listed corrosive irritating vapors toxic if ingested causes staining on skin flammable none listed Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 2 of 7 (d) If any of these get in your eyes, what is the proper response? First: Next: (e) What should be done with all waste generated in the titrations lab? corrosive irritating vapors toxic if ingested causes staining on skin flammable none listed Neutralize the acid with a sodium bicarbonate solution. Flush the affected area with a lot of water. Notify the instructor about the accident. Neutralize the acid with a sodium bicarbonate solution. Have your lab partner notify the instructor about the spill while you continue rinsing with water. Flush the affected area with some more water. They should be thrown in the trash can. They should be flushed down the sink with plenty of water. There will be no waste material in this experiment. They should be sent to the cafeteria for recycling. They should be disposed of in the labeled container. Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 3 of 7 Additional Materials Titrations 2. 3/3 points | Previous AnswersNCSUGenChem102LabV1 9.PRE.02. Green checks and red X's are not displayed for this question. The following statements concern techniques involving volumetric glassware used in the titrations experiment. (a) When the procedure says "condition the graduated cylinder with vinegar before using it", what does that mean? (b) A buret should be read to which nearest mL? (c) What should you do when filling a buret? (Select all that apply.) Rinse with vinegar solution several times. Wash thoroughly with soap and water. Dry the glassware completely before using it. Rinse with deionized water several times. Gently buff the outside with a wool cloth. 1 mL 0.001 mL 0.5 mL 0.05 mL 0.1 mL 0.01 mL Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 4 of 7 Additional Materials Titrations 3. 6/6 points | Previous AnswersNCSUGenChem102LabV1 9.PRE.03. Green checks and red X's are not displayed for this question. Answer the following questions relating to the titrations experiment. (Select all that apply.) (a) The buret in this titration experiment should contain which of the following? (b) The Erlenmeyer flask should contain which of the following at the beginning of the experiment? Condition the buret first. Fill the buret and drain if needed to below the zero mark. Make sure the stopcock is open. Fill the buret exactly to the zero mark. Make sure the stopcock is closed. Make sure there are no air bubbles left in the buret tip. deionized water NaOH solution phenolphthalein soapy water vinegar Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 5 of 7 (c) What is the stoichiometry of NaOH to vinegar in the neutralization reaction? (d) Choose all of the following that are true about the endpoint of the titration. Additional Materials Titrations deionized water NaOH solution phenolphthalein soapy water vinegar 1:3 1:2 1:1 2:1 3:1 The amount of analyte is half the amount of titrant added. The amount of analyte is twice the amount of titrant added. The solution will change to bright pink permanently. The amount of analyte equals the amount of titrant added. The solution will change to faint pink for at least 30 seconds. The solution will change to a clear solution for at least 30 seconds. Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 6 of 7 4. 3/3 points | Previous AnswersNCSUGenChem102LabV1 9.PRE.04. Green checks and red X's are not displayed for this question. The following statements concern techniques used in the titrations experiment. (Select all that apply.) (a) If you rinse your buret with deionized water, but do not condition with the titrant solution, what is the effect? (b) If you condition the receiver beaker with the analyte solution prior to adding the measured volume of analyte to be titrated, what is the effect? Additional Materials Titrations Volumetric Glassware a decrease in the volume of the titrant required to reach the end point an increase in the volume of the titrant required to reach the end point an overestimation of the number of moles of analyte present an underestimation of the number of moles of analyte present a decrease in the volume of the titrant required to reach the end point an increase in the volume of the titrant required to reach the end point an overestimation of the number of moles of analyte present an underestimation of the number of moles of analyte present Lab 9 PreLab - Titrations 11/25/14, 12:40 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120920 Page 7 of 7 5. 8/8 points | Previous AnswersNCSUGenChem102LabV1 9.PRE.05. You will perform several calculations with the data you obtain in the titrations lab. Below is an exercise to help you prepare. (a) If your titration solution is 0.416 M in NaOH, and the endpoint occurs at 13.90 mL of titrant, how many mmol of NaOH are required to reach the endpoint? 5.78 mmol NaOH (b) How many mmol of acetic acid (HC2H3O2) are required to react with the NaOH? 5.78 mmol HC2H3O2 (c) How many grams of acetic acid is this? .347 grams HC2H3O2 (d) If the mass of analyte is 10.15 grams, what is the mass % of acetic acid in the analyte? 3.42 % Additional Materials Titrations Periodic Table [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
 (1)ADQEFQEFEQ.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 06, 2022
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Oct 06, 2022
Downloads
0
Views
40








.png)





.png)
IIUUKK.png)
.png)
 (1)CDWDCW.png)

.png)
.png)
.png)
 (1)ACADC.png)