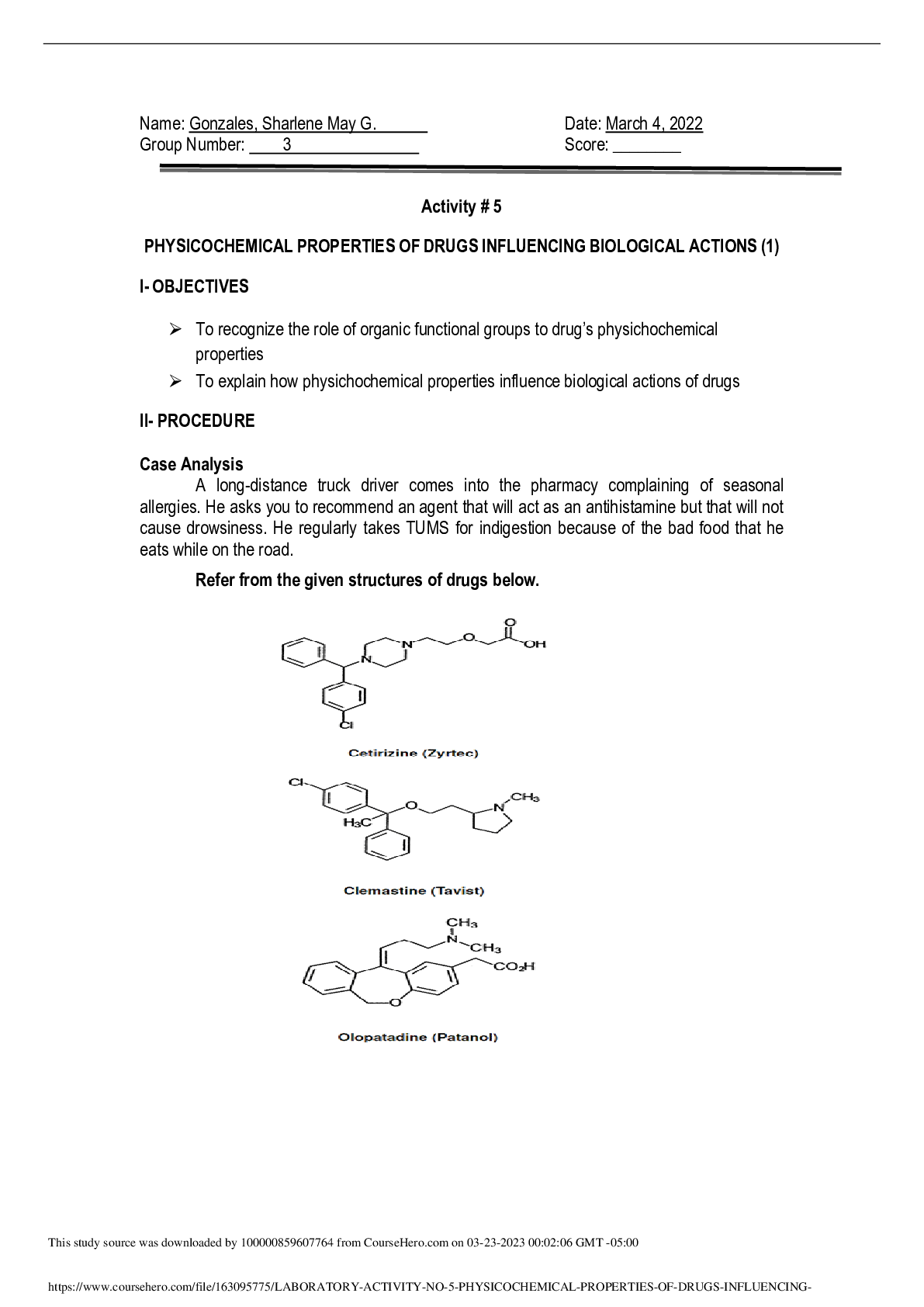
Chemistry > Lab Report > University of Cincinnati, Main Campus CHEM 111 Chem21Labs exp 3 (All)
University of Cincinnati, Main Campus CHEM 111 Chem21Labs exp 3
Document Content and Description Below
Chem21Labs http://www3.chem21labs.com/student2/?mode=assign&assignment=188 1/6 About Chem21Labs | Home | Contact Us | Reference Material | Logout Vamsidhar Student Area Vamsidhar is currently logg... ed in. This assignment is due at 11:45 PM on 09/29/2015. Any work submitted between that time and 11:45 PM on 09/30/2015 will result in an automatic 10 point deduction. Also, any work submitted between 11:45 PM on 09/30/2015 and 11:45 PM on 10/01/2015 will result in an additional 10 points being deducted for the assignment. PDF Lab Guide Due date passed. Please check with your Instructor or TA to submit lab work. Online Lab Submission Complete. Check back several days after the due date to view points added by your instructor. Experiment 3 Report Sheet SYNTHESIS OF AN ALUM KAl(SO4)2•12H2O Total Points = 124 150 Introduction Note: All essay answers throughout the semester must be composed in the html text editor below. If you copy / paste from another source, you may accidentally insert code that will make your lab sheet display improperly for your TA / Instructor (alerting them that the information was copied from some source). If you need to copy / paste from WORD, first copy / paste into a program like NotePad and then copy paste into the text area below. 6 10 The objective of the experiment is to obtain the theoretical and actual yield of the reaction. It will be achieved by dissolving Al by reaction with KOH. Converting Al(OH)4 To Al3+ by Reaction with H2SO4. Precipitating KA(SO4)2 12H2O by cooling, collecting, washing, and drying KAI(SO4)212H2O. [lab techniques? Experimental Procedure 3 3 The procedure followed was that given in the hand out downloaded from chem21labs.com Exp 3. Synthesis of an Alum pp 31. to 38 Mass of Al foil used .588 g Moles of Al used .0218 .0218 6 6 moles Volume of 3 M KOH used 20 mL Moles of KOH used .06 .06 6 6 moles Mass of KOH used 3.36 3.36 6 6 g Volume of 6 M H2SO4 used 16 mL Moles of H2SO4 used .096 .096 6 6 moles Mass of H2SO4 used 9.41 9.41 6 6 [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 25, 2022
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Sep 25, 2022
Downloads
0
Views
61
.png)



.png)
.png)














 (1).png)