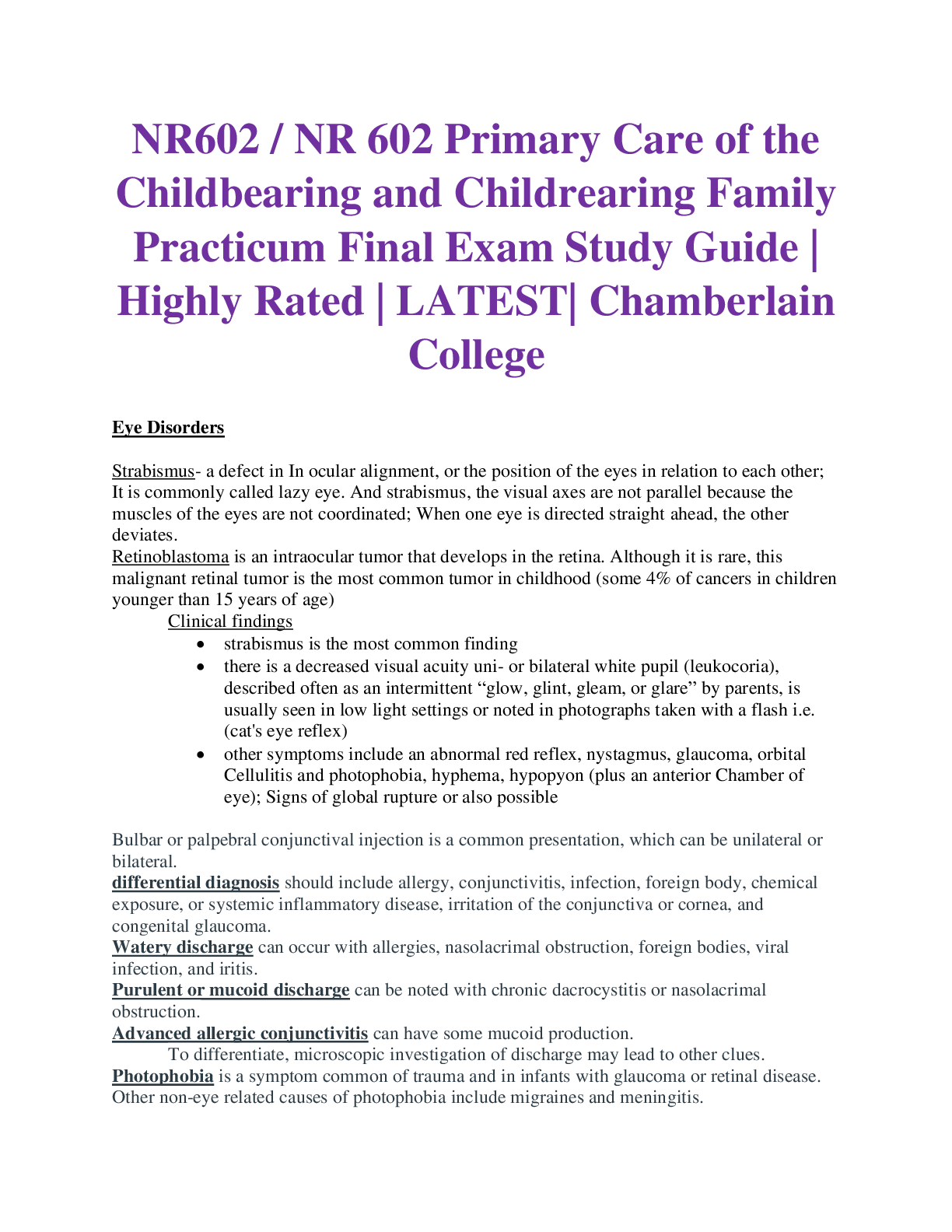
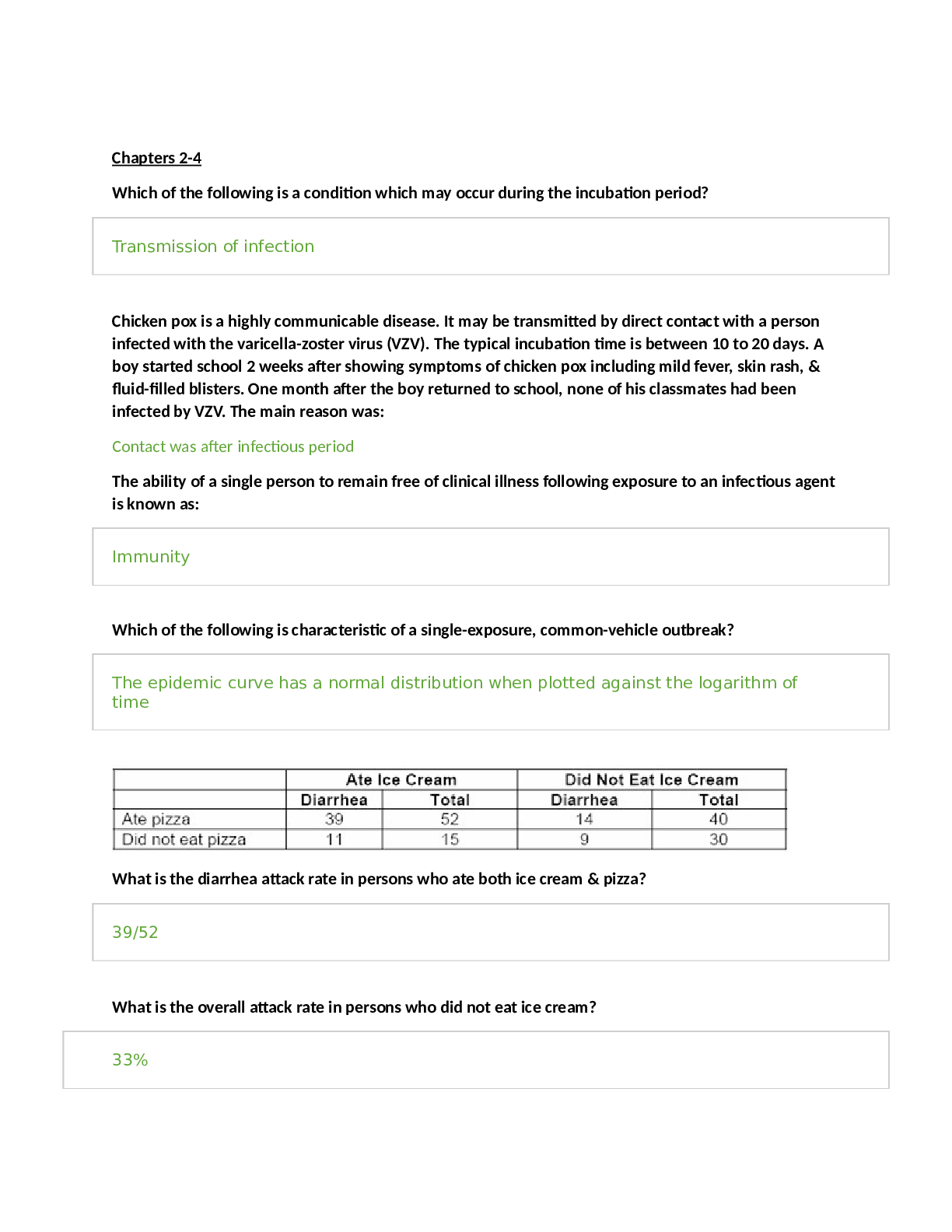
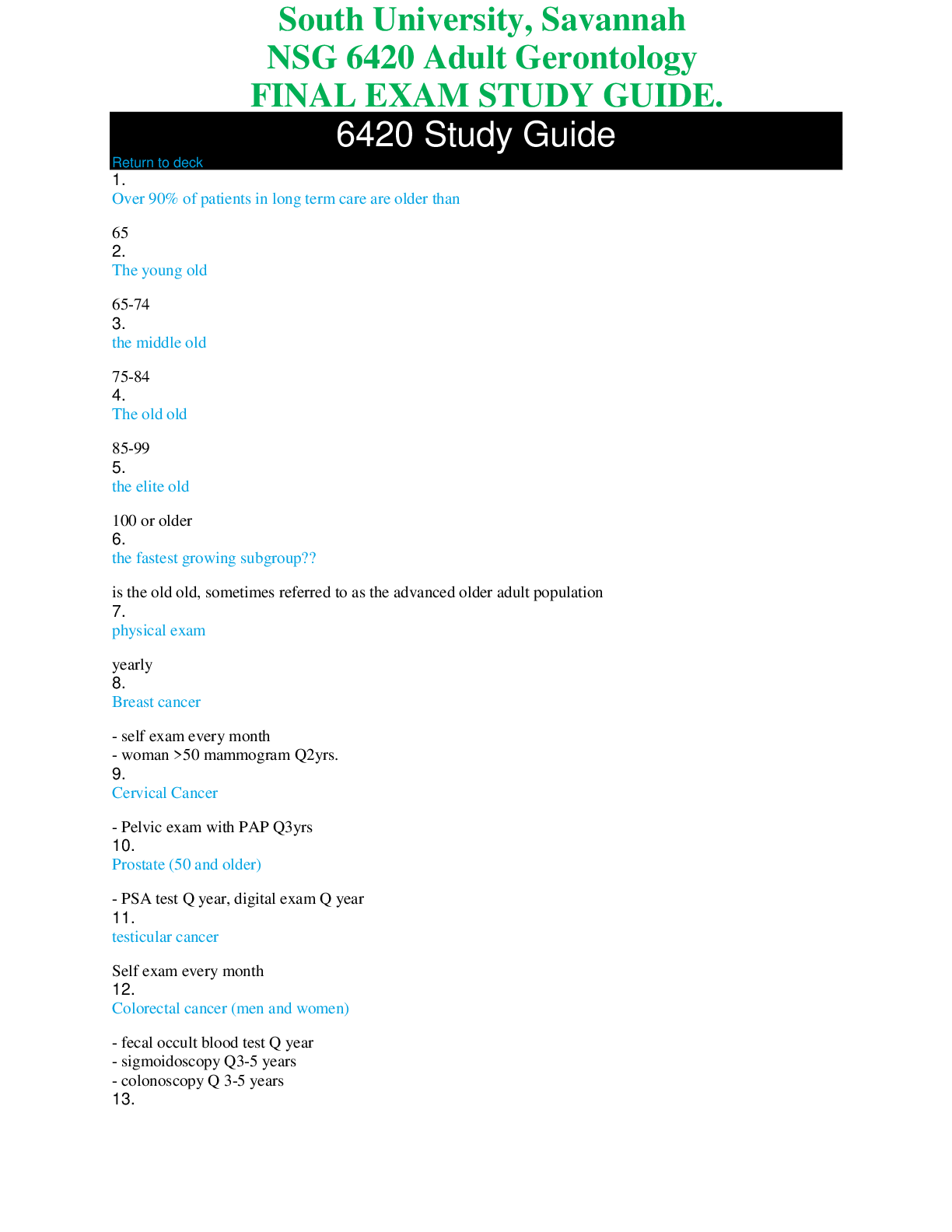
*NURSING > STUDY GUIDE > NR 507 FINAL EXAM STUDY GUIDE / NR 507 Week 8 Final Exam Study Guide, Complete Solutions A Guide (All)
NR 507 FINAL EXAM STUDY GUIDE / NR 507 Week 8 Final Exam Study Guide, Complete Solutions A Guide
Document Content and Description Below
NR507 Week 8 Final Exam Study Guide Reproductive: Endometrial cycle and the occurrence of ovulation: During the midfollicular phase, increasing levels of estrogen contribute to endometrial repair ... and proliferation, thus increasing endometrial thickness (luteal phase). Once ovulation occurs and serum progesterone levels increase, the endometrial tissue develops secretory characteristics (secretory phase). If implantation of a fertilized ovum does not take place, endometrial tissue begins to break down approximately 11 days after ovulation (ischemic phase of menstruation) (see Fig. 24.9). Sloughing of tissue (menstrual bleeding) begins about 14 days after ovulation. uterine prolapse; A uterine prolapse is when the uterus descends toward or into the vagina. Prevention of constipation and treatment of chronic cough may help prevent uterine prolapse, the uterus slips down into or protrudes out of the vagina. 1. Cause = pelvic floor muscles and ligaments stretch and weaken, providing inadequate support 2. Risks - aging/gravity, pregnancy/birthing, straining 3. Treatment - nothing to pessary to hysterectomy polycystic ovarian syndrome excessive androgens that affect follicular decline by suppressing apoptosis, enabling follicles, which normally disintegrate to survive, infertility testicular cancer and conditions that increase risk; Firm, nontender testicular mass cancer is a germ cell tumor arising from the male gamete Most common cancer in men ages 15-34 Incidence higher in Caucasians 5:1 (Caucasian:African American) - undescended testes - first-born - pre/perinatal estrogen exposure - polyvinyl cholirde exposure - advanced maternal age - Down's syndrome - Klinefelter's syndrome (XXY) - CIS - HIV/AIDS symptoms that require evaluation for breast cancer; painless lump signs of premenstrual dysphoric disorder; Premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) are the cyclic recurrence (in the luteal phase of the menstrual cycle) of distressing physical, psychologic, or behavioral changes that impair interpersonal relationships or interfere with usual activities and resolve after menstruation. Emotional symptoms, particularly depression, anger, irritability, and fatigue, have been reported as the most prominent and the most distressing, whereas physical symptoms seem to be the least prevalent and problematic. Physical symptoms include breast tenderness, abdominal bloating, headache, and swelling of extremities. In addition, underlying physical or psychologic disease may be aggravated premenstrually and must be diagnosed and treated independently from PMS/PMDD. dysfunctional uterine bleeding; The clinical manifestations of a woman include the following: irregular or heavy bleeding, passage of large clots, and depletion of iron stores. This person is experiencing: pathophysiology of prostate cancer; normal prostate epithelium --> proliferative inflammatory atrophy --> prostatic intraepithelial neoplasia --> localized prostate cancer --> metastatic prostate cancer --> androgen-independent cancer, More than 95% of prostatic neoplasms are histologically similar to adenocarcinomas and rely on androgen-dependent signaling for their development and progression.108-110 Most of these neoplasms occur in the periphery of the prostate. Prostatic adenocarcinoma is a heterogeneous group of tumors with a diverse spectrum of molecular and pathologic characteristics, and therefore clinical behaviors and challenges.111 The biologic aggressiveness of the neoplasm appears to be related to the degree of differentiation rather than the size of the tumo HPV and the development of cervical cancer Endocrine: body’s process for adapting to high hormone levels; D) down-regulation. To adapt to high levels of hormones, some cells have the capacity to decrease the number of receptors for that hormone through the process of down-regulation. Cushing’s Syndrome; B) ectopic production of ACTH from a lung tumor. Cushing disease is excessive ACTH production most commonly caused by an adrenal adenoma or a non-pituitary adenoma as is often seen with lung cancer. Autoimmune destruction of the adrenal cortex results in hypocortisolism or Addison disease. Cushing syndrome occurs whenever there is an excessive level of cortisol regardless of cause. Excessive production of aldosterone from a tumor in the adrenal cortex causes hyperaldosteronism. Cushing disease is excessive cortisol secondary to increased ACTH. causes of hypoparathyroidism; Parathyroid gland injury or removal Parathyroid gland injury or surgical removal of the gland is the most common cause of hypoparathyroidism. lab results that point to primary hypothyroidism; Primary hypothyroidism Low levels of T3 and T4 production caused by the destruction or removal of the thyroid gland (primary hypothyroidism) stimulate the anterior pituitary to increase the production of TSH. An endocrinologist orders a series of lab tests to assess thyroid function. Low levels of thyroid hormone (T3 and T4) and high levels of thyroid-stimulating hormone (TSH) are indicative of: pathophysiology of thyroid storm; Fever and tachycardia leading to high-output heart failure High levels of thyroid hormone in conjunction with high levels of stress hormones lead to fever, tachycardia, and eventually high-ouput heart failure if the condition is not treated. signs of thyrotoxicosis; Weight loss and enlarged thyroid gland Weight loss and enlarged thyroid gland are common signs of hyperthyroidism in thyrotoxicosis Neurological: dermatomes; Sensory innervation by a single spinal nerve in the skin is ___ A dermatome is the area of the skin of the human anatomy that is mainly supplied by branches of a single spinal sensory nerve root. These spinal sensory nerves enter the nerve root at the spinal cord, and their branches reach to the periphery of the body. The sensory nerves in the periphery of the body are a type of nerve that transmits signals from sensations (for example, pain symptoms, touch, temperature) to the spinal cord from specific areas of our anatomy. substance release at the synapse; Chemicals, called neurotransmitters, are released from one neuron at the presynaptic nerve terminal. Spondylolysis; structural defect either degenerative or developmental of the vertebra. heredity and associated with other malformations of the spine location of the motor and sensory areas of the brain; motor: Location: Precentral gyrus of frontal lobe of each hemisphere sensory: Location: Postcentral gyrus of the parietal lobe pathophysiology of cerebral infarction and excitotoxins; In the pathophysiology of cerebral infarction, the release of which substance is associated with neuron hyperpolarization and seizure activity? excitotoxins c. release of excitotoxins especially aspartate and glutamate - these are normal neurotransmitters that are released in toxic amounts during brain injury of any kind agnosia; Agnosia is the inability to process sensory information. Often there is a loss of ability to recognize objects, persons, sounds, shapes, or smells while the specific sense is not defective nor is there any significant memory loss. accumulation of blood in a subarachnoid hemorrhage; A) in the cerebrospinal fluid (CSF) between the brain and skull. most common cause of meningitis; Viral infections are the most common cause of meningitis Neisseria Meningitides (meningococcus) and Streptococcus pneumoniae (pnemococcus) Genitourinary: diet and the prevention of prostate cancer; Epidemiologic studies have found total fat intake, animal and saturated fat, red meat, and dairy products are associated with an increase in prostate cancer risk. Healthy lifestyles Diet low in red meats and high in vegetable and fruits Exercise Impact of Benign Prostatic Hypertrophy (BPH) on the urinary system -kidney disorders caused by pressure and back flow of urine - post renal -recurrent UTI's -pyelonephritis -sepsis-infection gets in blood -secondary renal insufficiency Genetics: the role of DNA in genetics; DNA stores, copies and transmits the genetic information in a cell. transcription; RNA is formed from DNA in a process that is called transcription and requires the enzyme RNA polymerase. RNA serves as the bridge between DNA and protein synthesis. effects of genetic mutations; -Any type of mutation in DNA or RNA can result in a nonfunctional protein or one with reduced function -A silent point mutation does not change protein function, a missense point mutation usually reduces protein function, and a nonsense mutation often eliminates protein function Trisomy; [BLANK_AUDIO] -Trisomy 21 -A person has 47 chromosomes with an extra chromosome in the 21 position -Due to nondisjunction, or the failure of chromosome pairs to separate properly during meiosis -This leads to a sperm or egg cell to have 24 chromosomes (instead of 23) -After conception, the extra chromosome is replicated in each cell in the embryo When we fertilize these abnormal gametes with normal gametes, what we will end up with is then a mixture of chromosomes. Extra amounts trisomy, cuz our gamete had two chromosomes instead of the one it should have. So we bring in the gamete with the matching chromosome. And now we have two plus one is a trisomy. In this case, the gamete didn't get the chromosome it needed. And so, the normal gamete that fertilizes it will not have a mate. So, we have monosomy. And when we have nondisjunction, the overall effect in meiosis will always be significant. Because this results in aneuploidy. Gamete cells that do not contain 23 chromosomes, the resulting embryo will have fewer or extra chromosomes. And not all of the abnormal embryos may even survive. [BLANK_AUDIO] When we have aneuploidy of the sex chromosome, during meiosis. This results in conditions such as Klinefelter syndrome, or Turner syndrome. Where we have an abnormal amount of the sex chromosomes, either too few or too many. Abnormalities are aneuploidy of sex chromosomes are always gender specific. So an individual with Klinefelter syndrome, even though they have two X chromosomes, the normal genotype for a female, that Y chromosome will still cause development of male characteristics. So Klinefelter syndrome that individual is genetically male. In the case of Turner syndrome that individual is missing one of the sex chromosomes. And so they have still one X chromosome. They will be typically female. But with a genetic abnormalities present. An employee of autosomal chromosomes results, it conditions such as Down syndrome trisomy 21 or Edwards syndrome Trisomy 18. Since these don't involve the sex chromosomes. These are not gender specific meaning that they can occur in either male or female. Down Syndrome; Down syndrome, a trisomy of chromosome 21, is the most well-known disease caused by a chromosome aberration. It affects 1 in 800 live births and is much more likely to occur in the offspring of women older than 35 years of age. Klinefelter syndrome; Klinefelter syndrome smallness of testes with fibrosis and hyalinization of seminiferous tubules, variable degrees of masculinization, azoospermia, infertility, and increased levels of urinary gonadotropins; associated typically with an XXY chromosome complement although variants include XXYY, XXXY, and XXXXY. also known as 47,XXY or XXY, is the set of symptoms that result from two or more X chromosomes in males. The primary features are infertility and small testicles. Often, symptoms may be subtle and many people do not realize they are affected. ... Klinefelter syndrome usually occurs randomly. diseases that have multifactorial traits; They include isolated cleft lip and/or cleft palate (CL/P), neural tube defects (anencephaly, spina bifida), clubfoot (talipes), and some forms of congenital heart disease. multifactorial inheritance; When environmental factors are also believed to cause variation in the trait, which is usually the case, the term multifactorial trait is used When the trait/disease is the consequence of the combined effect of mutations in multiple genes +/- environmental effects. Usually do not show a clear pattern of mendelian inheritance. The more genes that combine to produce the trait the more possible phenotypes there are resulting in a normal distribution within the population. low heritability (most affected progeny have unaffected parents), relationship by blood slightly increases the risk for an affected offspring, risk of affected relatives falls off very quickly with the degree of relationship, risk increases with the number of affected offspring in a family (indicated parents more likely to be closer to the threshold), a more severely affected parent is more likely to produce an affected offspring. Duchenne muscular dystrophy; Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by progressive muscle degeneration and weakness. It is one of nine types of muscular dystrophy. DMD is caused by an absence of dystrophin, a protein that helps keep muscle cells intact. first affecting the muscles of the hips, pelvic area, thighs and shoulders, and later the skeletal (voluntary) muscles in the arms, legs and trunk. The calves often are enlarged. Neurofibromatosis Neurofibromatosis is a genetic disorder that causes tumors to form on nerve tissue. These tumors can develop anywhere in your nervous system, including your brain, spinal cord and nerves. Neurofibromatosis is usually diagnosed in childhood or early adulthood Musculoskeletal: ions that initiate muscle contraction; Calcium growth of long bones in children; takes place in the epiphyseal plate. bones belonging to the appendicular skeleton; Ilium Immunity/Inflammation: how vaccines are formed; B) an attenuated antigen, a dead antigen, a detoxified toxin. populations at risk for getting systemic fungal infections and parasitic infections; immunocompromised systemic manifestations of infection; interleukin 1? Fever (pyrexia), Leukocytosis, & increase plasma protein synthesis wich are called acute phase reactants. mechanisms responsible for the increase in antimicrobial resistance worldwide; the misuse and overuse of antimicrobials is accelerating this process functions of normal flora in the body; The functions of the normal flora include digestion of substrates, production of vitamins, stimulation of cell maturation, stimulation of the immune system, aid in intestinal transit and colonization resistance desensitization therapy; improves allergies by which of the following mechanisms? Producing antibodies that prevent the allergen from binding to IgE cells involved in “left shift” in the WBC count differential; Bands and PMN's forms of immunity; If a person has innate resistance to a disease, the person has _____ immunity. A) natural If a person has resistance to a disease from natural exposure to an antigen, the person has which form of immunity?D) Active naturally acquired Humoral immunity is generated through the process of:C) producing antibodies. What type of immunity is conferred when an individual is given a vaccine? C) Active acquired immunity major histocompatibility class I antigens; are found in All body cells except for red blood cells inflammatory chemicals blocked by anti-inflammatory drugs; prostaglandins characteristics of acute phase reactant C-reactive protein A) Produced by the liver, B) Plasma indicator of inflammation, C) Significant risk factor for heart disease Dermatology: process by which a deep pressure ulcer heals; Secondary intention Successful healing requires continued adequate relief of pressure; débridement of dead tissue; opening of deep pockets for drainage; and repair of damaged tissue by construction of skin flaps for large, deep ulcers. Negative-pressure wound healing is used in the treatment of advanced-stage pressure injuries, but clinical trials are lacking.13 Infection requires treatment with topical and/or systemic agents, but there are no clear guidelines.14 Pain must be controlled. Randomized controlled trials are needed to determine the best methods for treating pressure injuries. Deep injuries develop closer to the bone as a result of tissue distortion and vascular occlusion from pressure that is perpendicular to the tissue (over the heels, trochanter, and ischia). Bacteria colonize the dead tissue, and infection is usually localized and self-limiting. Proteolytic enzymes from bacteria and macrophages dissolve necrotic tissues and cause a foul-smelling discharge that resembles, but is not, pus. The necrotic tissue initiates an inflammatory response with potential pain, fever, and leukocytosis. If the ulceration is large, toxicity and pain lead to a host of possible complications, including loss of appetite, debility, local/systemic infections, and renal insufficiency complications of the development of contractures during wound healing Excessive wound contraction may result in a deformity or contracture. Burn wounds are especially susceptible to the development of contractures. Internal contractures may occur as well, and are common in cirrhosis of the liver. Internally, scar tissue that becomes contracted constricts blood flow that may contribute to the development of portal hypertension and esophageal varices. Other types of internal contraction deformity include duodenal strictures caused by dysfunctional healing of an ulcer and esophageal strictures caused by chemical burns. Proper positioning and range-of-motion exercises, as well as surgery, are among the physical means used to overcome the excessive myofibroblast-derived tension that results in contractures. Clinical use of pharmacologic methods for control of wound contracture is still largely experimental, but includes control of myofibroblast contraction by the administration of smooth muscle cell inhibitors such as colchicine and inhibition of proper collagen matrix assembly with drugs that prevent either collagen cross-linking or MMP activity. These latter treatments are based on the knowledge that myofibroblast binding to collagen can “lock” contracted cells into position. Acid/Base: • causes of respiratory alkalosis; hyperventilation (anxiety, aspirin overdose) • Respiratory alkalosis occurs when there is alveolar hyperventilation and decreased concentration of plasma carbon dioxide (termed hypocapnia), thus increasing the ratio of HcO3 to PCO2 (H2CO3). Stimulation of ventilation is precipitated by hypoxemia (i.e., high altitudes); hypermetabolic states such as fever, anemia, and thyrotoxicosis; early salicylate intoxication; or anxiety or panic disorder. Improper use of mechanical ventilators can cause iatrogenic respiratory alkalosis. Secondary respiratory alkalosis may develop from hyperventilation stimulated by metabolic acidosis, causing a mixed acid-base disorder. Stress[1] • Pulmonary disorder[2] • Thermal insult[5] • High altitude areas[6] • Salicylate poisoning (aspirin overdose)[6] • Fever[1] • Hyperventilation (due to heart disorder or other, including improper mechanical ventilation)[1][7] • Vocal cord paralysis (compensation for loss of vocal volume results in over-breathing/breathlessness).[8] • Liver disease[6] molecules that act as buffers in the blood; Buffering occurs in response to changes in acid-base status. Buffers can absorb excessive H+ (acid) or hydroxyl ion (OH−) (base) to minimize fluctuations in pH. The buffer systems are located in both the ICF and the ECF compartments, and they function at different rates. Buffer systems exist as buffer pairs, consisting of a weak acid and its conjugate base (Table 3.11). The most important plasma buffer systems are bicarbonate–carbonic acid and hemoglobin. Phosphate and protein are the most important intracellular buffers and provide a first line of defense. Ammonia and phosphate can attach hydrogen ion and are important renal buffers. The carbonic acid–bicarbonate buffer pair operates in both the lung and the kidney. The greater the carbon dioxide partial pressure (PCO2), the more carbonic acid is formed. The buffer systems of the body also act to stabilize the acid-base balance (pH). There are three major buffer systems, with the plasma buffer system being the quickest to act (within seconds), whereas the respiratory system responds in minutes. The renal system is more powerful but responds over a period of hours to days. Each buffer system has a differing ability to compensate or correct acid-base disturbances. For example, respiratory compensation for a primary metabolic acid-base disorder can be initiated within minutes. However, the respiratory system can compensate for a metabolic disorder but requires the kidneys to fully correct the imbalance. Chronic respiratory acid-base disturbances can be fully compensated by renal activity, but the ultimate correction occurs through the regulation of carbon dioxide. Cardiovascular: most common cardiac valve disease in women; Mitral valve prolapse when myocardial ischemia may be reversible; Anaerobic metabolism maintains basic cellular integrity for approximately 20 minutes, although cardiac output during this time can be dramatically reduced. Individuals with reversible myocardial ischemia present clinically in several ways. Chronic atherosclerotic coronary obstruction usually results in recurrent predictable chest pain called stable angina. Abnormal vasospasm of coronary vessels results in unpredictable chest pain called Prinzmetal angina. Myocardial ischemia that does not cause detectable symptoms is called silent ischemia. . Reversible myocardial ischemia presents clinically in several ways. Chronic coronary obstruction results in recurrent predictable chest pain called stable angina. Abnormal vasospasm of coronary vessels results in unpredictable chest pain called Prinzmetal angina. Myocardial ischemia that does not cause detectable symptoms is called silent ischemia. Unstable angina causes reversible myocardial ischemia and is a harbinger of impending infarction. MI results when prolonged ischemia causes irreversible damage to the heart muscle. Sudden cardiac death can occur in any of the acute coronary syndromes. Reversibility of damage to ischemic myocardium after reperfusion may mainly occur within 60-min ischemia symptoms of stable angina; Severe substernal pain that lasts more than a few hours Severe substernal pain lasting more than a few hours is a symptom of a myocardial infarctioin. Stable angina manifests with chest tightness or discomfort that goes away with rest. Angina pectoris is chest pain caused by myocardial ischemia. Stable angina is caused by gradual luminal narrowing and hardening of the arterial walls, so that affected vessels cannot dilate in response to increased myocardial demand associated with physical exertion or emotional stress. With rest, blood flow is restored and no necrosis of myocardial cells results. Angina pectoris is typically experienced as transient substernal chest discomfort, ranging from a sensation of heaviness or pressure to moderately severe pain. Individuals often describe the sensation by clenching a fist over the left sternal border. The discomfort may be mistaken for indigestion. The pain is caused by the buildup of lactic acid or abnormal stretching of the ischemic myocardium that irritates myocardial nerve fibers. These afferent sympathetic fibers enter the spinal cord from levels C3 to T4, accounting for the variety of locations and radiation patterns of anginal pain. Pain may radiate to the neck, lower jaw, left arm, and left shoulder or occasionally to the back or down the right arm. Pallor, diaphoresis, and dyspnea may be associated with the pain. The pain is usually relieved by rest and nitrates. Myocardial ischemia in women may not present with typical angina. Common symptoms in women include atypical chest pain, palpitations, sense of unease, and severe fatigue. In addition, it is estimated that half of women with stable angina do not have obstructive coronary artery disease, but rather have microvascular angina that results from vasoconstriction of small coronary arterioles deep in the myocardium116 (see What's New? Women and Microvascular Angina). Similarly, in individuals who have autonomic nervous system dysfunction, such as older adults or those with diabetes, angina may be mild, atypical, or even silent (see the following). orthostatic hypotension; Stands up Orthostatic hypotension refers to a decrease in blood pressure upon standing and is caused by the gravitational changes on the circulation that are inadequately compensated for. The term orthostatic (postural) hypotension (OH) refers to a decrease in systolic blood pressure of at least 20 mmHg or a decrease in diastolic blood pressure of at least 10 mmHg within 3 minutes of moving to a standing position.55 Primary OH is often called neurogenic and is usually the result of neurologic disorders that affect autonomic function. Compensatory changes during standing normally increase sympathetic activity mediated through stretch receptors (baroreceptors) in the carotid sinus and the aortic arch (see Chapter 32). This reflex response to shifts in volume caused by postural changes leads to a prompt increase in heart rate and constriction of the systemic arterioles, which maintains a stable blood pressure. These compensatory mechanisms are not effective in maintaining a stable blood pressure in individuals with orthostatic hypotension. Primary OH is often chronic. Older adults are susceptible to this type of OH because of slowing of postural reflexes as part of the aging process. It also occurs in neurologic diseases, such as Parkinson disease, multiple system atrophy, and inherited neurologic disorders. Multiple system atrophy is a severe form of chronic autonomic failure in which there are multiple central nervous system degenerative changes, and Parkinson disease. Individuals with this disorder also may exhibit supine hypertension, altered drug sensitivity, hyperresponsiveness of blood pressure to hypo/hyperventilation, sleep apnea, and other neurologic disturbances.56Primary OH is a significant risk factor for falls and associated injury. It also is associated with an increased risk of death, coronary artery disease, heart failure, and stroke.57 Secondary OH is often acute and associated with (1) altered body chemistry, (2) drug action (e.g., antihypertensives or antidepressants), (3) prolonged immobility caused by illness, (4) starvation, (5) physical exhaustion, (6) any condition that produces volume depletion (e.g., massive diuresis, potassium or sodium depletion), and (7) any condition that results in venous pooling (e.g., pregnancy, extensive varicosities of the lower extremities). Other more chronic forms of secondary OH are seen with adrenal insufficiency, diabetes mellitus, cardiovascular diseases, and paraneoplastic syndromes.57 Orthostatic hypotension often is accompanied by dizziness, blurring or loss of vision, and syncope or fainting. To assess hypotensive episode frequency, severity, and correlation with symptoms, 24-hour blood pressure monitoring is recommended. Basic diagnostic tests include ECG and blood electrolyte measurements. Other tests in selected individuals may include autonomic testing, serum catecholamine measurements, and heart rate variability testing. Treatment for secondary OH is focused on correcting the underlying disorder. No curative treatment is available for primary orthostatic hypertension. Management includes liberalizing salt intake; raising the head of the bed; wearing thigh-high stockings; and administering erythropoietin, somatostatin, volume expansion with mineralocorticoids, and vasoconstrictors, such as midodrine and pyridostigmine isolated systolic hypertension; Isolated systolic hypertension (ISH) is elevated systolic blood pressure accompanied by normal diastolic blood pressure (less than 90 mmHg). ISH is becoming more prevalent in all age groups and is strongly associated with cardiovascular and cerebrovascular events.5loss of elasticity of the arteries resulting in an increase in cardiac output or stroke volume, a systolic blood pressure consistently greater than 160 mmHg, and a diastolic pressure less than 90 mmHg. results of sustained controlled hypertension; Vascular remodeling Over time, prolonged vasoconstriction can result in permanent remodeling of blood vessel walls. Renal disease, stroke and retinal damage High pressures in the vasculature cause damage to many organs, including the eye (retinal injury), kidneys (nephrosclerosis), and brain (aneurysm resulting in stroke). In the healthy individual, the RAAS provides an important homeostatic mechanism for maintaining adequate blood pressure and therefore tissue perfusion (see Chapter 32). In hypertensive individuals, overactivity of the RAAS contributes to salt and water retention and increased vascular resistance. In the brain, angiotensin (ang) II enhances sympathetic neural outflow and alters the release of hormones that contribute to endothelial dysfunction, insulin resistance, dyslipidemia, and platelet aggregation.23 Further, ang II mediates arteriolar remodeling, which is a structural change in the vessel wall that results in permanent increases in peripheral resistance24 (see Figs. 33.5 and 32.28). Ang II is associated with end-organ effects of hypertension, including atherosclerosis, renal disease, and cardiac hypertrophy. Decreased renal salt excretion. (shift in pressure-natriuresis relationship) the relationship of insulin resistance on the development of primary hypertension; Insulin resistance activates the sympathetic nervous system, contributes to the development of diabetes, dyslipidemia, and eventually atherosclerosis, and promotes thrombus formation Insulin resistance contributes to increases in SNS activity, peripheral resistance, endothelial injury and thrombus formation. Finally, insulin resistance is common in hypertension, even in individuals without clinical diabetes. Insulin resistance is associated with endothelial injury and affects renal function, causing renal salt and water retention.37 Insulin resistance is associated with overactivity of the SNS and the RAAS. It is interesting to note that in many individuals with diabetes treated with drugs that increase insulin sensitivity, blood pressure often declines, even in the absence of antihypertensive drugs. The interactions between obesity, hypertension, insulin resistance, and lipid disorders in the metabolic syndrome result in a high risk of cardiovascular disease defects in the normal secretion of natriuretic hormones and the impact on renal system; Sodium Natriuretic hormones affect renal reabsorption of sodium. The natriuretic hormones modulate renal sodium (Na+) excretion and require adequate potassium, calcium, and magnesium to function properly. The natriuretic hormones include atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), C-type natriuretic peptide (CNP), and urodilatin. These hormones induce diuresis; enhancement of renal blood flow and glomerular filtration rate, systemic vasodilatation, and suppression of aldosterone; and inhibition of the SNS. Dysfunction of these hormones, along with alterations in the RAAS and the SNS, cause an increase in vascular tone and a shift in the pressure-natriuresis relationship. When there is inadequate natriuretic function, serum levels of the natriuretic peptides rise in an attempt to compensate. In hypertension, increased ANP and BNP levels are linked to an increased risk for ventricular hypertrophy, atherosclerosis, and heart failure.28 Salt retention leads to water retention and increased blood volume, which contributes to an increase in blood pressure. Subtle renal injury results, with renal vasoconstriction and tissue ischemia. Tissue ischemia causes inflammation of the kidney and contributes to dysfunction of the glomeruli and tubules and promotes additional sodium retention. Increasing dietary intake of potassium, calcium, and magnesium can enhance natriuretic peptide function. New natriuretic peptide agonists are being studied. effects of increased sympathetic nervous system activity due to primary hypertension; Peripheral vasoconstriction A major factor in the development of primary hypertension is vasoconstriction and increased peripheral resistance caused by abnormal SNS activity. The SNS contributes to the pathogenesis of hypertension in many people. In the healthy individual, the SNS contributes to the maintenance of adequate blood pressure and tissue perfusion by promoting cardiac contractility and heart rate (maintenance of adequate cardiac output) and by inducing arteriolar vasoconstriction (maintenance of adequate peripheral resistance). In individuals with hypertension, overactivity of the SNS can result from increased production of catecholamines (epinephrine and norepinephrine) or from increased receptor reactivity involving these neurotransmitters.22 Increased SNS activity causes increased heart rate and systemic vasoconstriction, thus raising the blood pressure. Efferent sympathetic outflow stimulates renin release, increases tubular sodium reabsorption, and reduces renal blood flow. Additional mechanisms of SNS-induced hypertension include structural changes in blood vessels (vascular remodeling), insulin resistance, increased renin and angiotensin levels, and procoagulant effects.22 The SNS is implicated in the cardiovascular and renal complications of hypertension. Beta-blocking medications oppose the effects of the SNS and have been used for decades in the treatment of hypertension. However, because of their side effects, these medications are no longer considered first-line treatment. The role of the SNS in the pathogenesis of cardiovascular disease is summarized in complications of unstable plaque in the coronary arteries; Myocardial infarction An unstable plaque can rupture, and the resulting thrombus can obstruct the lumen, causing an abrupt halt to myocardial blood flow. This event is called a myocardial infarction Many plaques, however, are “unstable,” meaning they are prone to rupture even before they affect blood flow and are clinically silent until they rupture. Plaque rupture occurs because of the inflammatory activation of proteinases (matrix metalloproteinases and cathepsins), apoptosis of cells within the plaque, and bleeding within the lesion (plaque hemorrhage).76,77 Plaques that have ruptured are called complicated plaques (see Fig. 33.12). Once rupture occurs, exposure of underlying tissue results in platelet adhesion, initiation of the clotting cascade, and rapid thrombus formation that may suddenly occlude the affected vessel, resulting in ischemia and infarction. Aspirin or other antithrombotic agents are used to prevent this complication of atherosclerotic disease. forms of dyslipidemia associated with the development of the fatty streak in atherosclerosis; High LDL In atherosclerosis, LDL adheres to the injured endothelium and is oxidized by macrophages to form the fatty streak. High serum LDL is a risk factor for atherosclerosis. Injured endothelial cells become inflamed and cannot make normal amounts of antithrombotic and vasodilating cytokines. The adventitia also plays an important role through production of reactive oxygen species and activation of endothelial inflammation.72 Low-density lipoprotein (LDL) penetrates into the subintima of arterial walls, where it is trapped by proteoglycans (Fig. 33.13). Inflammation, oxidative stress, and activation of macrophages cause the aggregated LDL to become oxidized. Hypertension, increased levels of LDL, oxidative stress, and activation of the renin-angiotensin-aldosterone system all contribute to an acceleration of this step in atherogenesis.73 Inflammation and oxidized LDL cause endothelial cells to express adhesion molecules that bind monocytes and other inflammatory and immune cells. Monocytes penetrate the vessel wall becoming macrophages. Several types of receptors on these macrophages (toll-like receptors [TLRs] and LDL receptor-related protein [LRP]) enable detection and engulfment of the oxidized LDL.74 These lipid-laden macrophages are now called foam cells, and when they accumulate in significant amounts, they form a lesion called a fatty streak and numerous inflammatory cytokines are released (e.g., tumor necrosis factor-alpha [TNF-α], interferons, interleukins, and C-reactive protein), as well as enzymes that further injure the vessel wall.75 Growth factors also are released, including ang II, fibroblast growth factor, TGF-β, and platelet-derived growth factor, which stimulate smooth muscle cell proliferation in the affected vessel. These smooth muscle cells produce collagen and migrate over the fatty streak forming a fibrous plaque (see Fig. 33.12).71 The fibrous plaque may calcify, protrude into the vessel lumen, and obstruct blood flow to distal tissues especially during exercise, which may cause symptoms (e.g., angina or intermittent claudication). events that initiate the process of atherosclerosis; Endothelial injury and release of cytokines Endothelial injury and the subsequent release of cytokines are the events that initiate atherosclerosis. signs and symptoms of increased left atrial and pulmonary venous pressures in left sided heart failure; A) dyspnea and cough. differences between left and right sided heart failure; Of the following diseases, which is the most common cause of right heart failure? Left HF A patient is diagnosed with chronic pulmonary disease and elevated pulmonary vascular resistance. Which of the following heart failures generally results from this condition? RHF Systemic congestion. Jugular venous distension, hepatomegaly, peripheral edema. RHF Pulmonary congestion. Orthopnea, paroxysmal nocturnal dyspnea, pulmonary edema, pleuar effusion.LHF infective endocarditis Infective endocarditis (IE) is a general term used to describe infection and inflammation of the endocardium, especially the cardiac valves. The incidence of IE is increasing in the United States, in large part because of the increase in the implantation of prosthetic valves168 (Box 33.3). Bacteria are the most common cause of IE with Staphylococcus aureus the most common causative agent worldwide.169 Other causes include streptococci, enterococci, viruses, fungi, rickettsia, and parasites.168 The pathogenesis of IE requires at least three critical elements 1. Trauma, congenital heart disease, valvular heart disease, and the presence of prosthetic valves are the most common risk factors for endocardial damage that leads to IE. Turbulent blood caused by these abnormalities usually affects the atrial surface of atrioventricular valves or the ventricular surface of semilunar valves. Endocardial damage exposes the endothelial basement membrane, which contains a type of collagen that attracts platelets and thereby stimulates sterile thrombus formation on the membrane. This causes an inflammatory reaction (nonbacterial thrombotic endocarditis). 1. 2. Blood-borne microorganism adherence to the damaged endocardial surface. Bacteria may enter the bloodstream during injection drug use, trauma, dental procedures that involve manipulation of the gingiva, cardiac surgery, genitourinary procedures and indwelling catheters in the presence of infection, or gastrointestinal instrumentation, or may spread from uncomplicated upper respiratory tract or skin infections. Bacteria adhere to the damaged endocardium using adhesins. 2. 3. Formation of infective endocardial vegetations (Fig. 33.36). Bacteria infiltrate the sterile thrombi and accelerate fibrin formation by activating the clotting cascade. These vegetative lesions can form anywhere on the endocardium but usually occur on heart valves and surrounding structures. Although endocardial tissue is constantly bathed in antibody-containing blood and is surrounded by scavenging monocytes and polymorphonuclear leukocytes, bacterial colonies are inaccessible to host defenses because they are embedded in the protective fibrin clots. Embolization from these vegetations can lead to abscesses and characteristic skin changes, such as petechiae, splinter hemorrhages, and Janeway lesions (nonpainful hemorrhagic lesions on the palms and soles). Peripheral vascular disease: pathophysiology of deep vein thrombosis; The pathophysiology of deep venous thrombosis (DVT) can be described through three processes, known as Virchow's triad. Which of the following is not a component of Virchow's Triad? DVT is a blood clot that remains attached to a vessel wall, usually in a single side of a lower extremity (Fig. 33.2). A detached thrombus is a thromboembolus. Venous thrombi are more common than arterial thrombi because flow and pressure are lower in the veins than in the arteries. The American Heart Association (AHA) estimates that about 2 million people in the United States will have VTE annually with approximately 44,000 deaths.5 Three factors (termed the triad of Virchow) promote venous thrombosis: (1) venous stasis (associated with immobility, obesity, prolonged leg dependency, age, congestive heart failure [CHF]), (2) venous intimal damage (related to trauma, venipuncture, IV medications), and (3) hypercoagulable states (from inherited disorders, smoking, malignancy, liver disease, pregnancy, oral contraceptives, hormone replacement, hyperhomocysteinemia, antiphospholipid syndrome).6 Virtually everyone who is hospitalized is at significant risk for DVT, especially those with orthopedic trauma or surgery, spinal cord injury, age older than 60 years, and obstetric/gynecologic conditions. Individuals with malignancy (especially ovarian and pancreatic cancer), and women who are pregnant are also at significant risk. The most common heritable hypercoagulable states are abnormal factor V Leiden and prothrombin gene variant 20210A, both of which predispose patients to DVT.6 Other less common causes are deficiencies of the endogenous anticoagulants protein C, protein S, and antithrombin. Accumulation of clotting factors and platelets leads to thrombus formation in the vein, often near a venous valve. Inflammation around the thrombus promotes further platelet aggregation, and the thrombus grows proximally. Most thrombi eventually dissolve without treatment, but untreated DVT is associated with a high risk of thromboembolization of a part of the clot from the leg traveling to the lung resulting in a pulmonary embolism7 (see Chapter 36). In up to one-third of individuals with DVT, persistent venous outflow obstruction may lead to post-thrombotic syndrome (PTS) characterized by chronic, persistent pain; edema; and ulceration of the affected limb.8 Vichow’s triad Stasis [4]The first category, alterations in normal blood flow, refers to several situations. These include venous stasis, long surgical operations, prolonged immobility (whilst on a long plane or car ride, bed bound during hospitalization), and varicose veins. The equivalence of Virchow's version and the modern version has been disputed. Endothelial injuryor vessel wall injuryThe second category, injuries and/or trauma to endothelium includes vessel piercings and damages arising from shear stress or hypertension. This category is ruled by surface phenomena and contact with procoagulant surfaces, such as bacteria, shards of foreign materials, biomaterials of implants or medical devices, membranes of activated platelets, and membranes of monocytes in chronic inflammation. HypercoagulabilityThe last category, alterations in the constitution of blood,[6] has numerous possible risk factors such as hyperviscosity, coagulation factor V Leiden mutation, coagulation factor II G2021A mutation, deficiency of antithrombin III, protein C or S deficiency, nephrotic syndrome, changes after severe trauma or burn, cancer, late pregnancy and delivery, race, advanced age, cigarette smoking, hormonal contraceptives, and obesity. All of these risk factors can cause the situation called hypercoagulability (excessively easy clotting of blood). Hematology: physiological response to hypoxia in anemia; Hypoxemia: Renal hypoxia caused by the decreased oxygen-carrying capacity of erythrocytes in anemia stimulates the production of erythropoietin. populations at the highest risk for developing folate deficiency anemia; Alcoholics: Alcoholics are at high risk for folate deficiency anemia as a result of malnutrition and impairment of folate metabolism. causes of iron deficiency anemia The causes of IDA include (1) dietary deficiency, (2) impaired absorption, (3) increased requirement, and (4) chronic blood loss. Impaired absorption can occur in sprue, disorders involving alterations of fat absorption, and chronic diarrhea.4 Other causes for both genders include use of medications that cause GI bleeding (such as aspirin or nonsteroidal antiinflammatory drugs [NSAIDs]); surgical procedures that decrease stomach acidity, intestinal transit time, and absorption (e.g., gastric bypass); and eating disorders, such as pica, which is the craving and eating of nonnutritional substances, such as dirt, chalk, and paper. expected lab test results found in long standing iron deficiency anemia; normal serum b12, folate, bili: high: free erythrocyte, total iron binding Sickle Cell Anemia; One of the cardinal features of sickle cell anemia includes acute and chronic dysfunction of which organs? (Select all that apply.) a. Spleen b. Bones c. Brain d. Lungs Hemoglobin reacts to deoxygenation or dehydration by sickling the erythrocytes: Sickle cell disease results in the formation of abnormal hemoglobin (Hgb S) that reacts to deoxygenation or dehydration by stiffening and elongating the erythrocyte into a "sickled" shape. Sickle cell disease does not affect platelets. causes of aplastic anemia; Bone marrow destruction of infiltration: The term aplastic anemia is somewhat of a misnomer because bone marrow failure affects the production of erythrocytes, white blood cells, and platelets. Viral infection: epsiten barr, HIV, drugs, industrial toxins, & radiation exposure. underlying pathophysiologic mechanisms leading to autoimmune hemolytic anemia; Antigen-antibody reaction: Autoimmune hemolytic anemias are caused by an antibody-antigen reaction on the surface of red blood cells. secondary polycythemia; caused by hypoxemia at high altitude manifests with which of the following problems? Increased erythrocyte production: In response to hypoxia, increased erythropoietin secretion stimulates bone marrow production of erythrocytes, resulting in a high hematocrit and hyperviscosity of the blood. anemia of chronic renal failure; Decreased erythropoietin: Anemia associated with chronic renal failure is caused by decreased erythropoietin secretion secondary to damaged renal tubular epithelial cells and decreased erythrocyte life span as a result of uremia. Fluid and Electrolytes: conditions that result in pure water deficit (hypertonic volume depletion); ) Hyperventilation caused by fever B) Inability to concentrate the urine C) Coma osmoreceptors that stimulate thirst and the release of ADH; hypothalamus Osmoreceptors, which detect an increase in blood osmolarity, stimulating the release of antidiuretic hormone, which stimulates thirst, are found in the hypothalamus. causes of hypernatremia; B) decreased antidiuretic hormone secretion. effects of increased aldosterone; B) renal retention of sodium and water. dependent edema; Edema that occurs in the feet and legs when standing or around the sacrum and buttocks while lying down is called: definition of isotonic; Which state describes the ideal proportion of electrolyte-to-water content in the body? principle of capillary oncotic pressure; The inward-pulling force of particles in the vascular fluid is called the: types of fluid compartments in the body; A) Intracellular- holds the most B) Interstitial C) Intravascular D) Peritoneal extracellular Pulmonary: most effective measure to prevent pulmonary embolus from developing in patients; A) Ambulate patients frequently to prevent blood clot formation. Venous stagnation, a major risk factor for deep vein thrombus and pulmonary embolism formation, can be prevented by frequent ambulation after surgery. The ideal treatment of PE is prevention through risk factor recognition and elimination of predisposing factors. Venous stasis in hospitalized individuals is minimized by bed exercises, frequent position changes, early ambulation, and pneumatic calf compression. Most at-risk individuals also will receive prophylactic anticoagulation with low-molecular-weight heparin, warfarin, or fondaparinux. Oral anticoagulants that are not dependent on vitamin K levels, such as rivaroxaban, apixaban, and dabigatran, have been approved for the prevention and treatment of PE and DVT and do not require long-term anticoagulation monitoring.135,136 Once venous thromboembolism has occurred, rapid assessment of hemodynamic stability and oxygenation is essential. Low-molecular-weight heparin, unfractionated heparin, fondaparinux, or one of the new anticoagulants not dependent on vitamin K levels are indicated for stable individuals.131 In those with unstable hemodynamics, thrombolytic therapy, either intravenously provided or catheter directed, reduces mortality.132 Oxygen and hemodynamic stabilization with fluids may be needed for life-threatening complications. Surgical embolectomy is indicated in massive PE. After stabilization, anticoagulation therapy is continued for several months. In individuals who have contraindications to anticoagulation therapy, the placement of a filter in the inferior vena cava can prevent emboli from reaching the lungs. when the practitioner will note tactile fremitus; Consolidation (palpable chest vibrations), in PNA Tactile fremitus is normally more intense in the right second intercostal space, as well as in the interscapular region, as these areas are closest to the bronchial trifurcation (right side) or bifurcation (left side). Tactile fremitus is pathologically increased over areas of consolidation and decreased or absent over areas of pleural effusion or pneumothorax (when there is air outside the lung in the chest cavity, preventing lung expansion). tactile fremitus. a tremulous vibration of the chest wall during speaking that is palpable on physical examination. Tactile fremitus may be decreased or absent when vibrations from the larynx to the chest surface are impeded by chronic obstructive pulmonary disease, obstruction, pleural effusion, or pneumothorax. “Tactile fremitus increases in intensity whenever the density of lung tissue increases, such as in consolidation or fibrosis, and will decrease when a lung space is occupied with an increase of fluid or air (e.g., pleural effusion, pneumothorax and emphysema). The causes of increased tactile fremitus include: Pneumonia, Lung tumor or mass, Pulmonary fibrosis, Atelectasis. cause of acute airway obstruction in the patient with chronic bronchitis; inflammation, bronchial smooth muscle hypertrophy, and production of thick, tenacious mucus. types of pneumothorax; A client experiences an increased pressure in the chest cavity resulting in a collapse of the right lung and is diagnosed with a secondary pneumothorax. This diagnosis is based on what criterion? The client experienced some type of chest trauma resulting in the symptoms. __secondary______ pneumothorax indicates there was trauma to the chest resulting in the collapse of the lung. A ____tension______ pneumothorax causes a deviation of the trachea. ____tension_______ is a life-threatening condition caused by trapping of air in the pleural space, producing displacement of the great vessels and heart. _spontaneous_______ pneumothorax has no known cause. A __primary______ pneumothorax results from the rupture of blebs. Primary (spontaneous) pneumothorax occurs unexpectedly in healthy individuals (usually men) between ages 20 and 40 years and is caused by the spontaneous rupture of blebs (blister-like formations) on the visceral pleura. Smoking is a risk factor.14 Bleb rupture can occur during sleep, rest, or exercise. The ruptured bleb or blebs are usually located in the apexes of the lungs. The cause of bleb formation is not known, although more than 80% of these individuals have been found to have emphysema-like changes in their lungs even if they have no history of smoking or no known genetic disorder. Approximately 10% of affected individuals have a significant family history of primary pneumothorax that has been linked to autosomal dominant mutations in the folliculin gene, which influences cell-to-cell adhesion and cell growth and causes cystic lung disease and kidney tumors (Birt-Hogg-Dubé syndrome).15 Ruptured blebs damage the visceral pleura and create a conduit for air to travel from the lower airways into the pleural space (bronchopleural fistulae). Secondary (traumatic) pneumothorax can be caused by chest trauma, such as a rib fracture, stab or bullet wounds, or a surgical procedure that tears the pleura; rupture of a bleb or bulla (larger vesicle) as occurs in COPD; or mechanical ventilation, particularly if it includes positive end-expiratory pressure (PEEP).16 Iatrogenic pneumothorax is most commonly caused by transthoracic needle aspiration.17 Primary and secondary pneumothorax can present as either open or tension. In open pneumothorax (communicating pneumothorax), air pressure in the pleural space equals barometric pressure because air that is drawn into the pleural space during inspiration (through the damaged chest wall and parietal pleura or through the lungs and damaged visceral pleura) is forced back out during expiration. In tension pneumothorax, however, the site of pleural rupture acts as a one-way valve, permitting air to enter on inspiration but preventing its escape by closing during expiration. As more and more air enters the pleural space, air pressure in the pneumothorax begins to exceed barometric pressure. Air pressure in the pleural space pushes against the already recoiled lung, causing compression atelectasis, and against the mediastinum, compressing and displacing the heart, great vessels, and trachea (Fig. 36.4). The pathophysiologic effects of tension pneumothorax are life-threatening. Clinical manifestations of primary or secondary pneumothorax begin with sudden pleural pain, tachypnea, and dyspnea. Depending on the size of the pneumothorax, physical examination may reveal absent or decreased breath sounds and hyperresonance to percussion on the affected side. Tension pneumothorax may be complicated by severe hypoxemia, tracheal deviation away from the affected lung, and hypotension (low blood pressure). Deterioration occurs rapidly and immediate treatment is required. Diagnosis of pneumothorax is made with chest radiographs, ultrasound, and computed tomography (CT). Pneumothorax is treated by aspiration, usually with insertion of a chest tube that is attached to a water-seal drainage system with suction. After the pneumothorax is evacuated and the pleural rupture is healed, the chest tube is removed. For individuals with persistent air leaks, other interventions may be needed including thoracoscopic surgical techniques or pleurodesis (instillation of a caustic substance into the pleural space). Heimlich valves (one-way valves connected to the end of the chest drain) provide an option for home care in persistent spontaneous pneumothorax. Smoking cessation is strongly advised.18 Pneumothorax the collapse of a lung and subsequent escape of air into the pleural cavity between the lung and the chest wall that is caused by trauma, environmental factors, or spontaneous occurrence and results in a sudden pain in the chest. Iatrogenic pneumothorax the presence of air or gas in the pleural space caused by a rupture in the visceral pleura (which surrounds the lungs) or the parietal pleura and chest wall; is most commonly caused by transthoracic needle aspiration. Open pneumothorax (communicating pneumothorax) spontaneous and traumatic pneumothorax in which air pressure in the pleural space equals barometric pressure because air that is drawn into the pleural space during inspiration (through the damaged chest wall and parietal pleura or through the lungs and damaged visceral pleura) is forced out during expiration. Primary (spontaneous) pneumothorax occurs unexpectedly in healthy individuals (usually men) between ages 20 and 40 years; is most often caused by the spontaneous rupture of blebs on the visceral pleura. Secondary (traumatic) pneumothorax spontaneous or secondary pneumothorax beginning with sudden pleural pain, tachypnea, and possibly mild dyspnea. Tension pneumothorax the site of pleural rupture acts as a one-way valve, permitting air to enter on inspiration, but preventing its escape by closing during expiration and leading to air pressure in the pneumothorax exceeding barometric pressure. results of the loss of alph-1-antitrypsin in emphysema; A patient is born with an α-antitrypsin deficiency. Which of the following conditions will most likely manifest? Primary emphysema Normally, α1-antitrypsin inhibits the action of many proteolytic enzymes; therefore individuals who have α1-antitrypsin deficiency increase their likelihood of developing emphysema because proteolysis in lung tissues is not inhibited. Homozygous individuals have a 70% to 80% likelihood of developing lung disease. α1-Antitrypsin deficiency is suggested in individuals who develop emphysema before 40 years of age and in those who do not smoke but still develop the disease. Affected individuals often develop emphysema, which is a lung disease caused by damage to the small air sacs in the lungs (alveoli). About 10 percent of infants with alpha-1 antitrypsin deficiency develop liver disease, which often causes yellowing of the skin and whites of the eyes (jaundice). Approximately 15 percent of adults with alpha-1 antitrypsin deficiency develop liver damage (cirrhosis) due to the formation of scar tissue in the liver. Signs of cirrhosis include a swollen abdomen, swollen feet or legs, and jaundice. Individuals with alpha-1 antitrypsin deficiency are also at risk of developing a type of liver cancer called hepatocellular carcinoma. the result of loss of surfactant in ARDS; Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) results from an acute, diffuse injury to the __ alveolocapillary ________ membrane and decreased__surfactant______ production, which increases membrane permeability and causes edema, atelectasis, and hypoxemia. Surfactant is inactivated, and its production by type II alveolar cells is impaired as alveoli and respiratory bronchioles fill with fluid or collapse. The intraalveolar hemorrhagic exudate becomes a cellular granulation tissue appearing as hyaline membranes and there is progressive hypoxemia. The lungs become less compliant, the work of breathing increases, ventilation of alveoli decreases, and hypercapnia develops. Acute respiratory distress syndrome (ARDS) is caused by many factors including inhalation of toxicants, acute barotrauma, acid aspiration, and burns. Surfactantfunction is impaired in ARDS and acute airway injury resulting in high surface tension with alveolar and small airway collapse, edema, hypoxemia, and death. Without normal surfactant, the tissue surrounding the air sacs in the lungs (the alveoli) sticks together (because of a force called surface tension) after exhalation, causing the alveoli to collapse. ... There are several types of surfactant dysfunction, which are identified by the genetic cause of the condition. Characteristics of Cheyne-Stokes respirations -Alternating periods of deep and shallow breathing -Ventilations that inc. in volume until peaked reached after which ventilations dec. to apnoea lasting 15-60s Breathing pattern has a smooth increase (crescendo) in the rate and depth of breathing (hyperpnea), which peaks and is followed by a gradual smooth decrease (decrescendo) in the rate and depth of breathing to the point of apnea when the cycle repeats itself. The hyperpneic phase lasts longer than the apneic phase (represents an amplitude change). Cheyne-Stokes respiration is an abnormal rhythm of ventilation (periodic breathing) with alternating periods of hyperventilation and apnea (crescendo-decrescendo pattern). In the damaged brain, higher levels of PaCO2 are required to stimulate ventilation, and increases in PaCO2 lead to tachypnea. The PaCO2 level then decreases to below normal, and breathing stops (apnea) until the carbon dioxide reaccumulates and again stimulates tachypnea (see Fig. 17.1). In cases of opiate or sedative drug overdose, the respiratory center is depressed and the rate of breathing gradually decreases until respiratory failure occurs. Cheyne-Stokes respiration an abnormal pattern of breathing in which tidal volume gradually increases followed by a gradual decrease and a period of apnea before returning to a normal respiratory pattern. Shock: causes of hypovolemic shock; too much fluid leaves the intravascular space dehydration hemorrhaging third-spacing large burns (caused by insufficient intravascular fluid volume). In hypovolemic shock, oxygen delivery is impaired by inadequate numbers of red blood cells or hemoglobin (which carries oxygen) and insufficient blood volume. Hypovolemic shock is caused by loss of whole blood (hemorrhage), plasma (burns), or interstitial fluid (diaphoresis, diabetes mellitus, diabetes insipidus, emesis, or diuresis) in large amounts. Loss of whole blood or plasma causes hypovolemia directly. Loss of interstitial fluid causes an indirect “relative” hypovolemia by promoting diffusion of plasma from the intravascular to the extravascular space. Hypovolemic shock begins to develop when intravascular volume has decreased by about 15%. Dehydration and trauma are the most common causes of hypovolemic shock in children. how the body maintains glucose levels during shock In shock, how does the body maintain blood glucose levels once available glucose and glycogen stores are used up? By breaking down protein to fuel gluconeogenesis Impaired glucose use can be caused by either impaired glucose delivery or impaired glucose uptake by the cells (see Fig. 49.1). The reasons for inadequate glucose delivery are the same as those enumerated for inadequate oxygen delivery. In addition, in septic and anaphylactic shock, glucose metabolism and consumption may be increased or disrupted because of fever or bacteria, and glucose uptake can be prevented by the presence of vasoactive toxins, endotoxins, histamine, and kinins. Some of the compensatory mechanisms activated by shock contribute to decreased glucose uptake and use by the cells. High serum levels of cortisol, growth hormone, and catecholamines account for hyperglycemia and insulin resistance, tachycardia, increased SVR, and increased cardiac contractility. Cells shift to glycogenolysis, gluconeogenesis, and lipolysis to generate fuel for survival (see Chapter 1). Except in the liver, kidneys, and muscles, the body's cells have extremely limited stores of glycogen. In fact, total body stores can fuel the metabolism for only about 10 hours. The depletion of fat and glycogen stores is not itself a cause of organ failure, but the energy costs of glycogenolysis and lipolysis are considerable and contribute to the cellular failure. Effective glucose level control in various shock states has been shown to improve outcomes. Hyperglycemia, caused by insulin resistance in the liver and muscle, is a common finding in the critically ill. This is most likely an adaptive response providing glucosefor the brain, cell nourishment and production, and wound healing; it also causes the increase in circulating corticosteroids produced in the stress response. The extent of appropriate glucose level control has been evaluated in recent years, leading to more aggressive treatment with continuous, titrating insulin infusions to maintain blood glucose levels closer to normal, thus promoting normal cellular function and healing.4 [Show More]
Last updated: 1 year ago
Preview 1 out of 21 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Dec 19, 2020
Number of pages
21
Written in
Additional information
This document has been written for:
Uploaded
Dec 19, 2020
Downloads
0
Views
35




















 Rasmussen College.png)