Chemistry > QUESTIONS & ANSWERS > Questions and Answers > VCE Chemistry Unit 1 Mole Concept Question & Answers Booklet (All)
Questions and Answers > VCE Chemistry Unit 1 Mole Concept Question & Answers Booklet
Document Content and Description Below
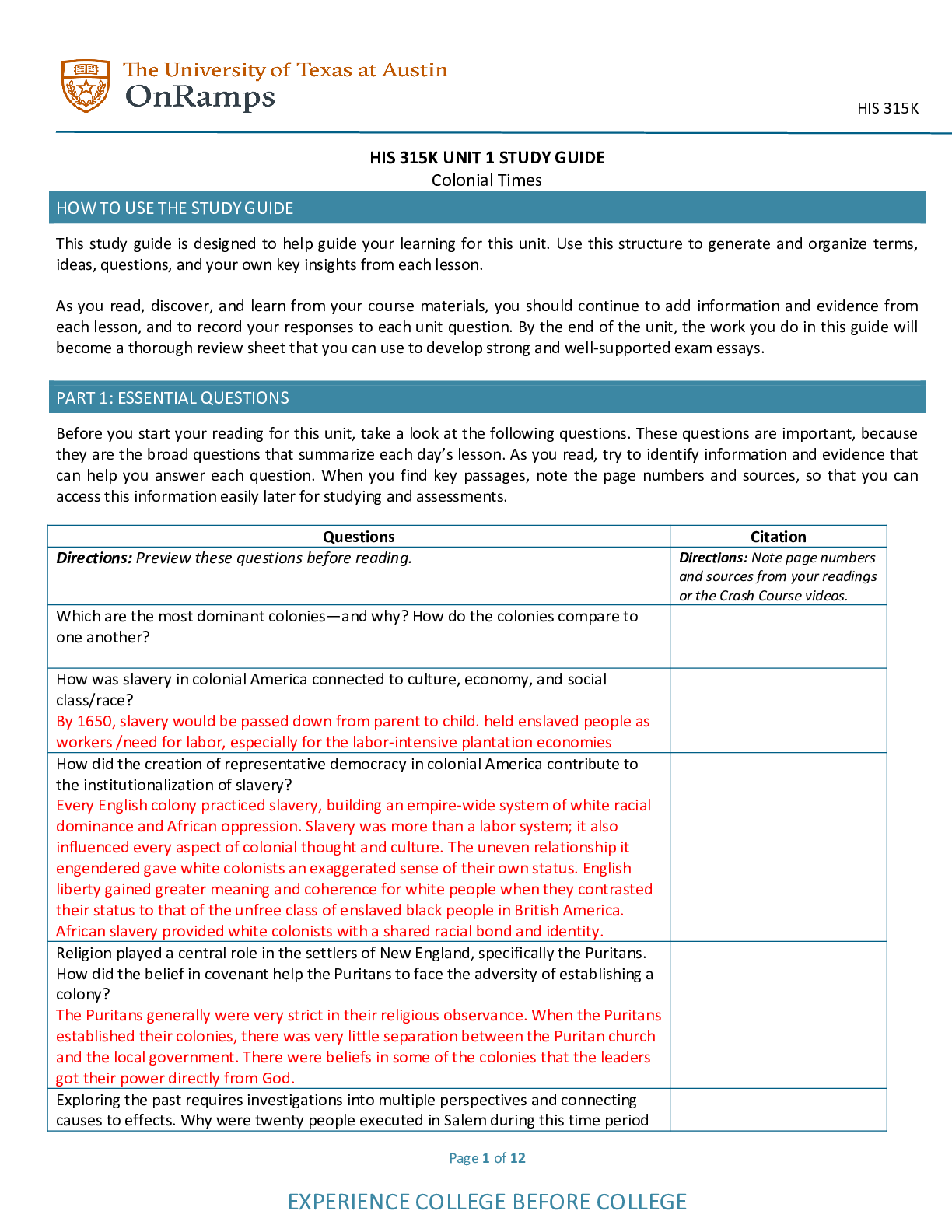
Wesley College CHE 123 VCE Chemistry Unit 1 Mole Concept Question Booklet Answers VCE Chemistry Wesley College Unit 1 Worksheet 1: Simple Mole Calculations No. Question Answer 1. Calculate the... number of : (a) electrons in 1.50 mol of electrons N(electrons) (b) atoms in 12.2 mol of zirconium atoms N(atoms7.34 ´ 1024 (c) atoms in 2.75 mol of CO2 molecules N(atoms) 1024 (d) ions in 0.034 mol of KI (potassium iodide) N(ions) 5. Calculate the amount (in mol) of: (a) atoms in 3.01 × 1023 zinc atoms n(atoms) = NNA = (b) molecules in 1.2 × 1010 H2O molecules n(molecules) = NNA = 1. 2×1010 6. 02×1023 = 2.0 × 10–14 mol (c) sodium ions in 1.25 × 1020 Na2O formula units n(Na+ ions) = NNA = 1. 25×1020 6. 02×1023 × 2 = 4.15 × 10–4 mol (d) atoms in 6.02 × 1021 glucose (C6H12O6) molecules n(atoms) = 9. Calculate the relative molecular mass of each of the following 13. Calculate the mass of one mole (molar mass) of each of the following 1 VCE Chemistry Wesley College Unit 1 No. Question Answer 17. Calculate the amount (in mol) of substance present in each of the following (a) 36 g of carbon = 0.20 mol = 21. Calculate the amount (in mol) of metal ions present in each of the following (a) 250 g of barium sulfate (BaSO4) n(BaSO4) = mM n(Ba2+ ions) = n(BaSO4) = 1.07 mol (b) 500 kg of iron(III) oxide (c) 0.926 g of potassium permanganate (KMnO4) n(KMnO4) = mM 25. Calculate the mass of each of the following: (a) 7.028 ´ 10–4 mol of potassium dichromate (K2Cr2O7) aspartame (C14H18N2O5) (d) 3.079 mol of calcium 2 VCE Chemistry Wesley College Unit 1 No. Question Answer (e) 0.0464 mol of ethanoic acid (CH3COOH) m(CH3COOH) = n ´ M = 0.0464 ´ 60.0 = 2.78 g 30. Calculate the amount (in mol) of hydrogen atoms in each of the following (a) 14.8 g of hydrogen nitrite mM (b) 35.0 kg of beryllium hydride mol (d) 0.977 g of aluminium hydrogenphosphate (Al2(HPO4)3) n(H) = 3n(Al2(HPO4)3) = 3 × mM 34. Calculate the mass of each of the following. (a) 2.50 × 1015 ethanol (C2H5OH) molecules m(C2H5OH) = A (c) 1.50 × 1024 hydrogen (H2) molecules VCE Chemistry Wesley College Unit 1 Worksheet 2: Mole Concept Multiple Choice Questions 4 VCE Chemistry Wesley College Unit 1 Worksheet 3: Percentage Composition VCE Chemistry Wesley College Unit 1 urea will supply the most nitrogen for a given mass of fertiliser 3. magnetite contains the greatest mass of iron VCE Chemistry Wesley College Unit 1 Worksheet 4: Empirical Formulas 1. (a) CH4 (b) NH2 (c) H2SO4 (d) CH2O 2. Empirical formula: SO3 3. Empirical formula: KMnO4 5. Empirical formula: C9H8O4 7 VCE Chemistry Wesley College Unit 1 6. Empirical formula: C3H2Cl 8 VCE Chemistry Wesley College Unit 1 Worksheet 5: Molecular Formulas 1. Mr(compound) = 88, Mr(C2H4O) = 44, so the ratio is The molecular formula is 2 2. M(hydrocarbon) 0.300 = 82.0 g mol–1 the molecular formula is hence the empirical formula is CH2O Mr(CH2O) = 30 = (60 ¸ 2), so the molecular formula is 2 ´ (CH2O) = C2H4O2 4. (a) Assume exactly 100 g of PABA. Thus, 61.31% C becomes 61.31 g C, 5.15% H becomes 5.15 g H, 10.21 g N becomes 10.21 g N and 23.33% O becomes 23.33 g O. Convert each of these masses to moles: Divide through by the smallest moles to find the ratio of C:H:N:O : The empirical formula of PABA is C7H7NO2 (b) The molecular formula is either the same as, or a multiple of the empirical formula. To find the multiple, one must compare the molar mass (MM) of the actual compound to the empirical formula weight (EFW). It is not necessary to use many significant figures to find this multiple. Calculate the EFW of PABA: 7(12 g/mol) + 7(1 g/mol) + 1(14 g/mol) + 2(16 g/mol) = 137 g/mol The molar mass of PABA is given to be 137 g/mol. Determine the multiple: The molecular formula for PABA is the same as the empirical formula: C7H7NO2 9 VCE Chemistry Wesley College Unit 1 5. (a) Assume exactly 100 grams of potassium ferricyanide. As result, 35.62 % K becomes 35.62 g K, Convert each of these masses to moles: Divide through by the smallest moles to find the ratio of K:Fe:C:N : The empirical formula for potassium ferricyanide is K3FeC6N6 (b) The molecular formula is either the same as, or a multiple of the empirical formula. To find the multiple, one must compare the molar mass (M) of the actual compound to the empirical formula weight (EFW). It is not necessary to use many significant figures to find this multiple. Calculate the EFW for potassium ferricyanide: The molar mass of potassium ferricyanide is given to be 329.1 g/mol. Determine the multiple: MM EFW The molecular formula is the same as the empirical formula: K3FeC6N6 6. (a) Assume exactly 100 g of lindane. As a result becomes 2.08 g H and 73.14% Cl becomes 73.14 g Cl. Convert each of these masses to moles: Divide through by the smallest moles to find the ratio of C:H:Cl : The empirical formula for lindane is CHCl. 10 VCE Chemistry Wesley College Unit 1 (b) The molecular formula is either the same as, or a multiple of the empirical formula. To find the multiple, one must compare the molar mass (M) of the actual compound to the empirical formula weight (EFW). It is not necessary to use many significant figures to find this multiple. Calculate the EFW: The molar mass of lindane is given to be 290 g/mol. Determine the multiple: The molecular formula of lindane is found by multiplying the subscripts of the empirical formula by the multiple of 6: [Show More]
Last updated: 1 year ago
Preview 1 out of 12 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 18, 2022
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Aug 18, 2022
Downloads
0
Views
34








.png)






.png)

.png)




.png)
.png)

.png)




.png)
.png)

