Chemistry > Experiment > Exploring the behavior of Gases (All)
Exploring the behavior of Gases
Document Content and Description Below
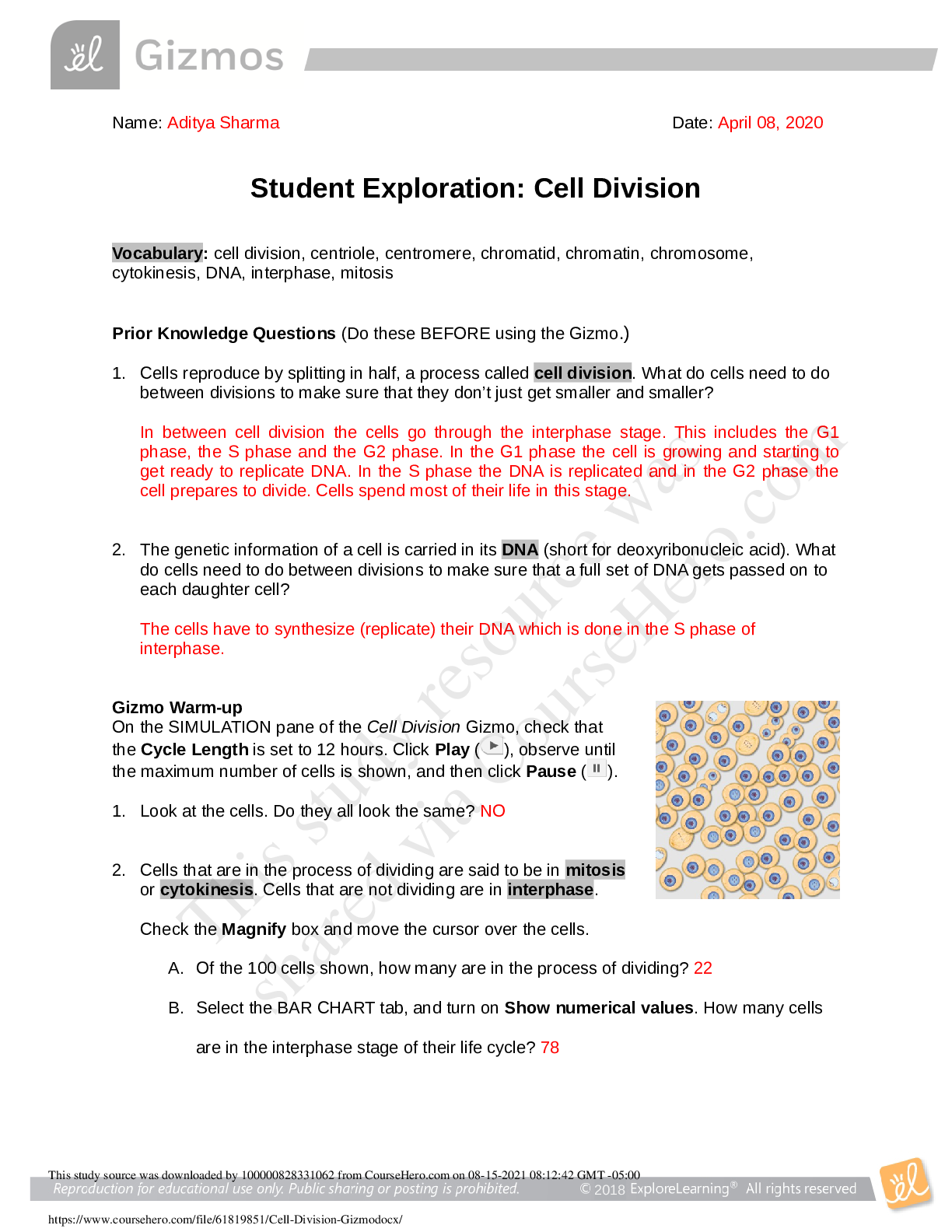
Part I 1. Go to the intro. Choose only one type of particle. 2. Record the pressure, temperature and volume. 3. Give one pump of gas and observe the behavior. How would you describe this? Record t... he pressure, temperature and volume. 4. Hold volume and temperature constant and give one more pump. Record the number of particles and the pressure in the data table below. Describe what you saw. Repeat this a few times, either increasing or decreasing the number of the same type of particles. Number of particles Pressure (atm) 5. Is there a relationship between the number of particles and the pressure? Briefly describe this. What is the pressure in the container due to? (what assumption are we making?) Modified resource from https://phet.colorado.edu/en/simulation/gases-intro 2 Part II: There are 3 parameters that need to be specified when describing a specific quantity of a gas. They are: Pressure, Volume and Temperature. We will keep the number of particles constant in each “experiment” and explore the effect (if any) a change in any of these parameters may have on the behavior of the gas. Choose the Laws option on the right. See picture Experiment 1- Volume 1. Give one pump of gas into the chamber. 2. Choose to hold the volume constant by selecting that option in the upper right-hand corner. See the picture. What is the initial temperature (in K) and pressure (in atm) in the chamber? 3. Use the slider at the bottom of the simulator to add heat and double the temperature. Did the pressure go up or go down? What is the new pressure in the chamber? 4. Keeping the volume constant (and the number of particles constant), change the temperature, and record the pressure. Repeat 4 times and record your data. Sketch a graph to the right of the table. Be sure to give a title to your graph and label the axis completely. Independent variable is : _________________ Dependent Variable: ____________________ Constants: ____________________________ Temperature (K) Pressure (atm) Describe the graph and relationship: This study source was downloaded by 100000830919685 from CourseHero.com on 06-06-2022 16:28:36 GMT -05:00 https://www.coursehero.com/file/59856218/Exploring-the-behavior-of-Gases-Phetpdf/Honors Chemistry Srikanthan Modified resource from https://phet.colorado.edu/en/simulation/gases-intro 3 Experiment 2 - Temperature 1. Reset the simulator by selecting the reset button in the bottom right corner of the simulation. 2. Give one pump of gas into the chamber. 3. Choose to hold the temperature constant by selecting that option in the upper right-hand corner. See the picture. What is the initial pressure (in atm) in the chamber? [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 06, 2022
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Aug 06, 2022
Downloads
0
Views
122









.png)

.png)