Chemistry > Lab Report > Veterans Memorial High SchoolCHEM 1312Lab Report - Solubility Equilibrium (All)
Veterans Memorial High SchoolCHEM 1312Lab Report - Solubility Equilibrium
Document Content and Description Below
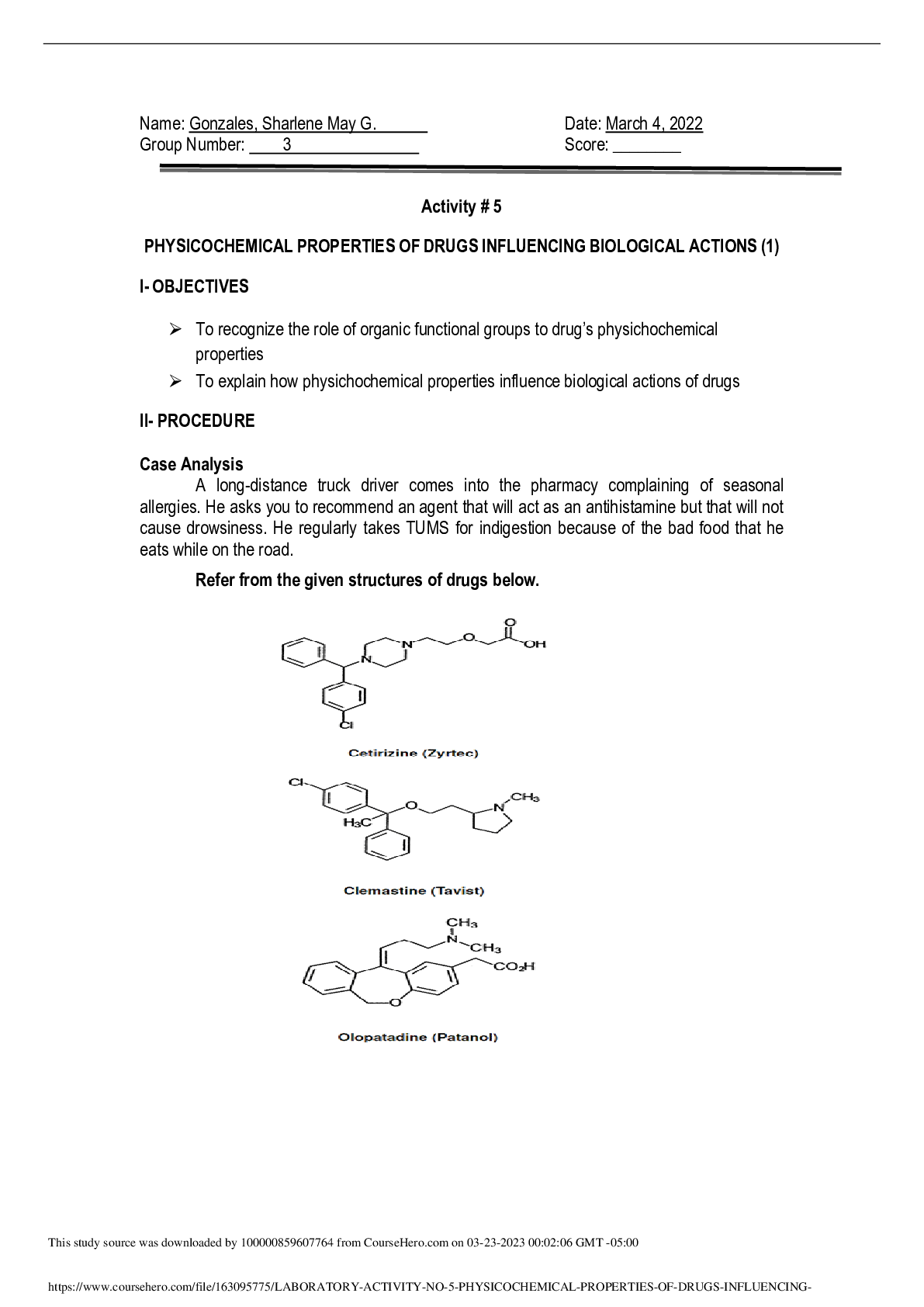
3.2.8 Lab 14: Solubility Equilibrium Lab Assignment AP Chemistry Sem 2 Name: ________________________ Points Possible: 50 Date: _____________ Adapted from: Quality Science Labs, Lab 14: Solubility ... Equilibrium Adapted from: ChemCollective. http://chemcollective.org/vlab/vlab.php DIRECTIONS: Background Ionic compounds such as salts, dissociate to form ions when they dissolve in water. An example is sodium chloride: (Equation 14.1) NaCl (s) → Na+ (aq) + Cl- (aq) Solid NaCl dissociates to become Na+ and Cl- ions. The Na+ cations are attracted to the negative “ends” of water molecules and the Cl- anions are attracted to the positive “ends” of water molecules. In other compounds the attraction between the cations and the anions is stronger and there is little dissociation. These are sparingly or slightly soluble salts. An example is silver chloride, AgCl. A beaker of this solution often has solid AgCl crystals in the bottom. The crystals are in equilibrium with the Ag+ and Cl- ions that are in solution: (Equation 14.2) AgCl (s) ←→ Ag+ (aq) + Cl- (aq) In this reaction the rate of AgCl dissolving is equal to the rate of Ag+ and Cl- ions combining to form solid AgCl. In other words, the reaction proceeds both ways at the same time and at the same rate. The system is said to be in equilibrium. The equilibrium constant is: (Equation 14.3) K = [Ag+ ][Cl-] AgCl(s) is not considered in the equilibrium constant because it is a solid. We call this special equilibrium constant the solubility product constant, Ksp. Some of the beautiful structures that you may have seen in caves are composed of sparingly soluble calcium carbonate. This material slightly dissolves in water and later precipitates when the water in the solution evaporates or the solution cools. Another sparingly soluble salt is calcium oxalate, which sometimes precipitates in our kidneys as kidney stones. These can be very painful and often require medical treatment. The solubility product constant for this is: (Equation 14.4) Ksp = [Ca+2][C2O4-2] Procedure Part I: Temperature and Solubility of Salts 1. Go to http://ir.chem.cmu.edu and click on “Virtual Lab” in the upper right-hand corner. To start, click the “Introductory Video and Support Information” li [Show More]
Last updated: 1 year ago
Preview 1 out of 4 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 07, 2021
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Jun 07, 2021
Downloads
0
Views
59

 (1).png)

.png)








.png)
.png)







 (1).png)






.png)

.png)

