Chemistry > TEST BANK > Package title: Solomons Test Bank Course TitleSolomons: (Nova Southeastern University - CHM 2211) CH (All)
Package title: Solomons Test Bank Course TitleSolomons: (Nova Southeastern University - CHM 2211) CHEMISTRY 11e Chapter Number: 11. This document contains 256 Questions and Answers.
Document Content and Description Below
1 Package title: Solomons Test Bank Course Title: Solomons 11e Chapter Number: 11 Question type: Multiple choice 1) What is the relationship between alcohols I and II? I II OH OH They are: a... ) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Easy 2) What is the relationship between alcohols I and II? I II OH OH They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C 2 Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Easy 3) What is the relationship between alcohols I and II? I II OH OH They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. E Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Easy 4) What is the relationship between alcohols I and II? They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. B 3 Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Easy 5) What is the relationship between alcohols I and II? They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. A Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Easy 6) What is the relationship between alcohols I and II? CH3 HO I II CH3 OH They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. B 4 Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Easy 7) What is the relationship between alcohols I and II? CH3 OH CH3 HO I II They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 8) What is the relationship between alcohols I and II? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. B 5 Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 9) What is the relationship between alcohols I and II? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 10) What is the relationship between alcohols I and II? CH3 OH HO CH3 I II They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. C 6 Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 11) What is the relationship between alcohols I and II? OH OH I II They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. B Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 12) What is the relationship between alcohols I and II? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. 7 A Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 13) What is the relationship between alcohols I and II? I II CH3 OH CH3 OH They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 14) What is the relationship between alcohols I and II? I II OH OH They are: a) conformers. b) constitutional isomers. 8 c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 15) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 16) What is the relationship between alcohols I and II? I II CH3 OH OH CH3 9 They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 17) What is the relationship between alcohols I and II? H CH3 OH H CH3 H H OH I II They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 18) What is the relationship between alcohols I and II? H CH3 OH H H CH3 H OH I II 10 They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.12 and 11.1 Difficulty Level: Medium 19) What is the relationship between alcohols I and II? CH3 HO I II CH3 HO They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.12 and 11.1 Difficulty Level: Medium 20) What is the relationship between alcohols I and II? 11 I II CH3 OH CH3 OH They are: a) different conformations of the same compound. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.12 and 11.1 Difficulty Level: Medium 21) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 12 22) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 23) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. D 13 Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 24) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. E Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 25) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. 14 C Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 26) What is the relationship between alcohols I and II? They are: a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. E Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 27) What is the relationship between alcohols I and II? They are: 15 a) conformers. b) constitutional isomers. c) enantiomers. d) diastereomers. e) identical. E Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 28) Which of the following can be described as “optically active, primary alcohol”? a) CH3CH2CH2CH2CH2OH b) (CH3)2CHCH2CH2OH c) CH3CH2CH(CH3)CH2OH d) (CH3)2CHCHOHCH3 e) Two of these choices. C Topic: Structure, Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Medium 29) What is the relationship between (1R,2S)-2-methylcyclopentanol and (1S,2R)-2- methylcyclopentanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. B Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 30) What is the relationship between (1R,2R)-2-methylcyclohexanol and (1R,2S)-2- methylcyclohexanol? They are: 16 a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 31) What is the relationship between (1S,2R)-2-methylcyclohexanol and (1R,2S)-2- methylcyclohexanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. B Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 32) What is the relationship between (1S,3R)-3-methylcyclohexanol and (1R,2R)-2- methylcyclohexanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. A Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 17 33) What is the relationship between (1R,2S)-2-methylcyclopentanol and (1S,2S)-2- methylcyclopentanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 34) What is the relationship between (1R,2S)-2-methylcycloheptanol and (1S,2R)-2- methylcycloheptanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. B Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 35) What is the relationship between (1R,2S)-2-methylcycloheptanol and (1R,2R)-2- methylcycloheptanol? They are: a) constitutional isomers. b) enantiomers. c) conformers d) diastereomers. e) identical. D Topic: Structure and Nomenclature Section: 5.3, 5.12 and 11.1 Difficulty Level: Hard 18 36) What is the correct IUPAC name for the following compound? CH3 CH3CHOHCHCHCH(CH3)2 CH3 a) 4-isopropyl-3,4-dimethyl-2-butanol b) 2,3,4-trimethyl-4-pentanol c) 1,1,2,3-tetramethyl-4-pentanol d) 3,4,5-trimethyl-2-hexanol e) 3,4,5,5-tetramethyl-2-pentanol D Topic: Nomenclature Section: 11.1 Difficulty Level: Easy 37) A correct IUPAC name for isobutyl alcohol is: a) 2-Methyl-1-propanol b) 2-Methyl-1-butanol c) 1-Methyl-1-propanol d) 1,1-Dimethyl-1-ethanol e) 3-Methyl-1-propanol A Topic: Nomenclature Section: 11.1 Difficulty Level: Easy 38) The IUPAC name of compound CH3CH2COH CH2CH3 CH2CH3 is: a) 1,1,1-Triethylmethanol b) 1,1-Diethyl-1-propanol c) 2-Ethyl-3-pentanol d) 3-Ethyl-3-pentanol e) tert-Heptanol 19 D Topic: Nomenclature Section: 11.1 Difficulty Level: Easy 39) What is the correct IUPAC name for the following compound? CH3CH2C=CCH2CH3 CH3 CH2CH2OH a) 3-methyl-4-ethyl-3-hexen-6-ol b) 4-ethyl-3-methyl-3,6-hexenol c) 3-ethyl-4-methyl-3-hexen-1-ol d) 3-methyl-4-(2-hydroxyethyl)-3-hexene e) 3-(2-hydroxyethyl)- 3-methyl-3-hexene C Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 40) What is a correct IUPAC name for the following compound? HO OH a) 1,2-butanediol b) isopropanol c) 1-propanol d) 1,2-propanediol e) Ethylene glycol D Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 20 41) What is a correct IUPAC name for the following compound? HO OH a) 1,2-butanediol b) isopropanol c) 1-proanol d) Propylene glycol e) 1,2-ethanediol E Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 42) The correct IUPAC name for tert-butyl alcohol is: a) 1-Butanol b) 2-Methyl-1-propanol c) 2-Methyl-2-propanol d) 2-Butanol e) 1,1-Dimethyl-1-ethanol C Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 43) 2,2-Dimethyl-1-propanol has the common name: a) Isoamyl alcohol b) Isopentyl alcohol c) tert-Pentyl alcohol d) Neopentyl alcohol e) 2-Methylisobutyl alcohol D Topic: Nomenclature Section: 11.1 21 Difficulty Level: Medium 44) Which of these, though often used, is an incorrect common name for CH3CHOHCH3? a) Isopropyl alcohol b) sec-Propyl alcohol c) 2-Propanol d) Isopropanol e) More than one of these choices. D Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 45) Which of these, though often used, is an incorrect common name for (CH3)3COH? a) tert-Butyl alcohol b) tert-Butanol c) 2-Methyl-2-propanol d) More than one is incorrect. e) Each is a correct name. B Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 46) Select the structure of benzyl methyl ether. 22 CH3 O CH3 CH3O CH3 O CH2 CH CH3 O CH CH3 CH3 CH2 O CH2 CH3 I II III IV V a) I b) II c) III d) IV e) V C Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 47) Which is a correct IUPAC name for CH3CH2OCH2CH2CH2OCH2CH3? a) 1,4-Dioxane b) Ethylene glycol diethyl ether c) 1,3-Diethoxypropane d) 1,2-Diethoxyethane e) 1,2-Diethoxymethane C Topic: Nomenclature Section: 11.1 Difficulty Level: Medium 48) The correct IUPAC substitutive name for OH is: 23 a) 4-Penten-2-methyl-2-ol b) 4-Methyl-1-penten-2-ol c) 2-Methyl-4-penten-2-ol d) 4-Methyl-1-penten-4-ol e) 4-Hydroxy-4-methyl-1-pentene C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 49) OH OH is properly named: a) cis-1,2-Cyclopentanediol b) meso-1,2-Cyclopentanediol c) (1R,2R)-1,2-Cyclopentanediol d) (1R,2S)-1,2-Cyclopentanediol e) (1S,2S)-1,2-Cyclopentanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 50) OH OH is properly named: a) 1,2-Cyclopentanediol b) 1,5-Cyclopentanediol c) (1R,2R)-1,2-Cyclopentanediol d) (1R,2S)-1,2-Cyclopentanediol e) (1S,2S)-1,2-Cyclopentanediol D Topic: Nomenclature Section: 11.1 24 Difficulty Level: Hard 51) OH OH is properly named: a) cis-1,2-Cyclohexanediol b) meso-1,2-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 52) is properly named: a) cis-1,2-Cyclohexanediol b) meso-1,2-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 53) is properly named: 25 a) cis-1,2-Cyclohexanediol b) meso-1,2-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 54) OH OH is properly named: a) 1,2-Cyclohexanediol b) 1,6-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol D Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 55) is properly named: a) 1,2-Cyclohexanediol b) 1,6-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol D 26 Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 56) is properly named: a) 1,2-Cyclohexanediol b) 1,6-Cyclohexanediol c) (1R,2R)-1,2-Cyclohexanediol d) (1R,2S)-1,2-Cyclohexanediol e) (1S,2S)-1,2-Cyclohexanediol D Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 57) OH OH is properly named: a) 1,3-Cyclopentanediol b) 1,4-Cyclopentanediol c) (1R,3R)-1,3-Cyclopentanediol d) (1R,3S)-1,3-Cyclopentanediol e) (1S,3S)-1,3-Cyclopentanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 27 58) HO OH is properly named: a) 1,3-Cycloheptanediol b) 1,4- Cycloheptanediol c) (1R,3R)-1,3- Cycloheptanediol d) (1R,3S)-1,3- Cycloheptanediol e) (1S,3S)-1,3- Cycloheptanediol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 59) HO OH is properly named: a) 1,4-Cycloheptanediol b) 1,5-Cycloheptanediol c) (1R,4R)-1,4-Cycloheptanediol d) (1R,4S)-1,4-Cycloheptanediol e) (1S,4S)-1,4-Cycloheptanediol D Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 60) A correct name for the following Fischer projection formula is: 28 OH H H3C C C CH3 a) (R)-3-Pentyn-2-ol b) (S)-3-Pentyn-2-ol c) (R)-2-Pentyn-4-ol d) (S)-2-Pentyn-4-ol e) (S)-2-Hydroxy-3-pentyne B Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 61) is properly named: a) 2-butanol b) Cyclobutanol c) bicyclo[1.1.1]butan-2-ol d) bicyclo[1.1.0]butan-2-ol e) bicyclo[1.1.0]butan-1-ol D Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 62) is properly named: a) bicyclo[1.1.1]pentan-2-ol b) Cyclopentanol c) 1-pentanol d) bicyclo[1.1.0] pentan-2-ol e) bicyclo[1.1.0] pentan-1-ol 29 A Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 63) is properly named: a) (2S)-bicyclo[1.1.1]octan-2-ol b) (2S)-bicyclo[2.2.2]octan-2-ol c) (2R)-bicyclo[2.2.2]octan-2-ol d) (1S)-bicyclo[2.2.2]octan-1-ol e) (2S)-bicyclo[2.2.1]octan-2-ol B Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 64) is properly named: a) (2S)-bicyclo[1.1.1]octan-2-ol b) (2S)-bicyclo[2.2.2]octan-2-ol c) (2R)-bicyclo[2.2.2]octan-2-ol d) (1S)-bicyclo[2.2.2]octan-1-ol e) (2S)-bicyclo[2.2.1]octan-2-ol C Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 30 65) is properly named: a) bicyclo[2.2.1]heptan-6-ol b) bicyclo[2.2.2]heptan-2-ol c) bicyclo[2.2.1]heptan-2-ol d) bicyclo[2.2.1]heptan-7-ol e) bicyclo[1.2.2]heptan-7-ol D Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 66) What is the most accurate name for the molecule represented by the following Fischer projection formula? H OCH3 CH3 H3CH2C a) sec-Butyl methyl ether b) Isobutyl methyl ether c) tert-Butyl methyl ether d) (R)-2-Methoxybutane e) (S)-2-Methoxybutane E Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 67) What is the total number of pentyl alcohols, including stereoisomers? a) 7 b) 8 c) 9 d) 10 e) 11 31 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 68) The number of primary alcohols corresponding to the formula C5H12O, counting stereoisomers separately, is: a) 1 b) 2 c) 3 d) 4 e) 5 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 69) The number of tertiary alcohols corresponding to the formula C5H12O, counting stereoisomers separately, is: a) 1 b) 2 c) 3 d) 4 e) 5 A Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 70) The number of secondary alcohols corresponding to the formula C5H12O, counting stereoisomers separately, is: a) 1 b) 2 c) 3 32 d) 4 e) 5 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 71) The total number of secondary alcohols corresponding to the formula C4H10O, counting stereoisomers separately, is: a) 1 b) 2 c) 3 d) 4 e) No secondary alcohols can be made from this formula B Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 72) The total number of secondary alcohols corresponding to the formula C6H14O, counting stereoisomers separately, is: a) 6 b) 8 c) 10 d) 12 e) 14 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 73) The total number of secondary alcohols corresponding to the formula C6H14O, ignoring stereoisomers, is: a) 2 33 b) 4 c) 6 d) 8 e) 10 C Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 74) The total number of primary alcohols corresponding to the formula C6H14O, counting stereoisomers separately, is: a) 2 b) 4 c) 6 d) 8 e) 10 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 75) The total number of primary alcohols corresponding to the formula C6H14O, ignoring stereoisomers, is: a) 4 b) 5 c) 6 d) 7 e) 18 D Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 76) The total number of ethers corresponding to the formula C5H12O, counting stereoisomers separately, is: 34 a) 4 b) 5 c) 6 d) 7 e) 8 D Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 77) The number of optically active pentyl alcohols (C5H11OH), i.e., the total number of individual enantiomers, is: a) 0 b) 2 c) 3 d) 4 e) 6 E Topic: Isomerism, Stereoisomers Section: 11.1 Difficulty Level: Medium 78) Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight.) a) CH3CH2CH2CH2CH3 b) CH3CH2CH2CH2OH c) CH3CH2CH2OCH3 d) CH3CH2CH2Cl e) CH3CH2OCH2CH3 B Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 35 79) Which compound would have the highest boiling point? a) CH3CH2CH2CH3 b) CH3CH2OCH3 c) CH3CH2CH2OH d) (CH3)2CHOH e) HOCH2CH2OH E Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 80) Which compound would have the lowest boiling point? a) CH3CH2CH2CH3 b) CH3CH2OCH2CH3 c) CH3CH2CH2OH d) (CH3)2CHOH e) HOCH2CH2OH A Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 81) Which compound would have the highest boiling point? a) CH3CH2CH2CH3 b) CH2(OH)CH(OH)CH2OH c) CH3CH2CH2OH d) (CH3)2CHOH e) HOCH2CH2OH B Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 82) Which compound would have the lowest solubility in water? 36 a) Diethyl ether b) Methyl propyl ether c) 1-Butanol d) 2-Butanol e) Pentane E Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 83) Which compound would have the greatest solubility in water? a) Diethyl ether b) Methyl propyl ether c) 1-Butanol d) 1,2-Butanediol e) Pentane D Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 84) Which compound would have the lowest boiling point? I II III IV V O OH OH CH2OH OH a) I b) II c) III d) IV e) V A Topic: Physical Properties, Comparison Section: 11.2 37 Difficulty Level: Easy 85) Which compound would have the lowest boiling point? a) I b) II c) III d) IV e) V A Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 86) Which compound would have the highest boiling point? I II III IV V O O OH OH O OH O O O O a) I b) II c) III d) IV 38 e) V E Topic: Physical Properties, Comparison Section: 11.2 Difficulty Level: Easy 87) Which of the following statements is NOT true of ethers? a) Ethers are generally unreactive molecules toward reagents other than strong acids. b) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight. c) Ethers generally have much lower water solubilities than alcohols with a corresponding molecular weight. d) Ethers can generally be cleaved by heating them with strong acids. e) Ethers form peroxides when allowed to stand in the presence of oxygen. C Topic: Ether Reactivity Section: 11.2 Difficulty Level: Easy 88) Which product(s) would you expect to obtain from the following sequence of reactions? CH3 CH3 OH CH3 OH CH3 H3C CH2OH O OH + enantiomer + enantiomer + enantiomer I II III IV V 1. BH3-THF 2. H2O2, NaOH ? a) I b) II c) III d) IV e) V C 39 Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Easy 89) Select the structure of the major product formed from the following reaction. CH3 CH3 OH CH3 OH CH2OH HO CH3 CH3 OH I II III IV V 1. Hg(OOCCH3)2 THF, H2O 2. NaBH4, NaOH ? a) I b) II c) III d) IV e) V D Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Easy 90) Anti-Markovnikov hydration of the carbon-carbon double bond occurs when an alkene reacts with: a) BH3:THF; then H2O2/OHb) BH3:THF; then CH3COOH c) Hg(OAc)2, THF, H2O; then NaBH4, OHd) Hg(OAc)2, THF, CH3OH; then NaBH4, OHe) Hg(OAc)2, THF, H2O; then BH3:THF A Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Easy 40 91) Which would be the best way to carry out the following synthesis? CH3CH2CHCH3 Br CH3CH2CH2CH2OH ? a) (1) HA, heat; (2) H3O+, H2O, heat b) (1) (CH3)3COK / (CH3)3COH; (2) BH3:THF, then H2O2, OHc) (1) (CH3)3COK / (CH3)3COH; (2) H3O+, then H2O, heat d) (1) KOH, C2H5OH; (2) BH3:THF, then H2O2, OHe) (1) KOH, C2H5OH; (2) HA, heat; (3) H3O+, H2O, heat B Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 92) Select the potential energy diagram that best represents the following reaction: H+ H2O OH 41 a) I b) II c) III d) IV e) V D Topic: Alcohol Synthesis, Mechanisms Section: 11.4 Difficulty Level: Medium 93) Which reaction can accomplish the following transformation in good yield: ? OH a) H+/H2O b) oxymercuration/demercuation c) hydroboration/oxidation d) Reaction with NaOH e) None of these choices 42 B Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 94) The hydroboration-oxidation procedure can be successfully employed for synthesis of deuterated derivatives, by using BD3 instead of BH3. What product would you expect from the following reaction? CH3 CH3 HO D H CH3 H D H CH3 BD2 D H CH3 D OH H CH3 H BD2 D 1. (BD3)2 2. H2O2/NaOH ? + enantiomer I + enantiomer II + enantiomer III + enantiomer IV + enantiomer V a) I b) II c) III d) IV e) V A Topic: Alcohol Synthesis, Isotope Labeling Section: 11.4 Difficulty Level: Medium 95) The oxymercuration-demercuration procedure can be successfully employed for synthesis of deuterated derivatives, by using NaBD4 instead of NaBH4 in the second step. What product would you expect from the following reaction? 43 1. Hg(OAc)2/H2O 2. NaBD4, NaOH ? OH OH D D OH OH D OH I II III IV V a) I b) II c) III d) IV e) V C Topic: Alcohol Synthesis, Isotope Labeling Section: 11.4 Difficulty Level: Medium 96) Which of the following would be a reasonable synthesis of 2-butanol? a) 1-Butene RCOOH b) 1-Butene 2. H2O2, NaOH 1. BH3-THF c) 1-Butene 2. NaBH4, NaOH 1. Hg(OAc)2, THF, H2O d) 1-Butene 1. Ha(OAc)2/CH3OH 2. NaBH4, NaOH e) None of these choices. 44 C Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 97) Which of the following would be a reasonable synthesis of CH3CH2CH2CH2OH? a) 1-Butene H3O+, heat b) 1-Butene 2. H2O2, NaOH 1. BH3-THF c) 1-Butene 2. NaBH4, NaOH 1. Hg(OAc)2, THF, H2O d) 1-Butene 1. Ha(OAc)2/CH3OH 2. NaBH4, NaOH e) None of these choices. B Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 98) Which would be the major product of the reaction shown? CH2CH3 H OH CH2CH3 H OH CH2CH3 H H H H CH2CH2OH H H H CH2CH3 OH CH2CH3 H 2. NaBH4, NaOH 1. Hg(OAc)2, THF, H2O ? O I II III IV V a) I b) II c) III 45 d) IV e) V B Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 99) What is the major product of the reaction: i) BH3, THF ii) H2O2,NaOH CH3 CH3 OH OH CH3 I II III IV CH3 OH ? + enantiomer + enantiomer a) I b) II c) III d) IV e) Both III and IV D Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 100) Oxymercuration-demercuration of 3-methylcyclopentene produces this/these product(s): I II III IV HO CH3 CH2OH CH3 CH3 OH OH a) I b) II 46 c) III d) IV e) Both III and IV E Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Medium 101) What is the major product of the reaction when (R)-3-methylcyclohexene is subjected to the hydroboration-oxidation sequence? a) (1R,2R)-2-methylcyclohexanol + (1S,2R)-2-methylcyclohexanol b) (1S,2R)-2-methylcyclohexanol + (1S,2S)-2-methylcyclohexanol c) (1R,3R)-3-methylcyclohexanol + (1S,3R)-3-methylcyclohexanol d) (1S,3R)-3-methylcyclohexanol (1S,3S)-3-methylcyclohexanol e) Two of these choices E Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 102) Which would be the best method for converting 3,3-dimethyl-1-pentene into 3,3-dimethyl- 2-pentanol? a) H3O+, heat b) BH3:THF; then H2O2, OHc) concd. H2SO4; then H2O, heat d) Hg(OAc)2/THF-H2O; then NaBH4,OHe) HBr; then NaOH/H2O D Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 103) Assuming an overall exothermic process, select the potential energy diagram that best represents the following reaction: 47 H+ H2O HO a) I b) II c) III d) IV e) V E Topic: Alcohol Synthesis, Mechanisms Section: 11.4 Difficulty Level: Hard 104) Select the structure of the major product formed from the following reaction. 48 H3O+ ? OH OH OH OH OH I II III IV V a) I b) II c) III d) IV e) V C Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 105) Select the structure of the major product formed from the following reaction. 49 H3O+ OH OH OH OH OH I II III IV IV ? a) I b) II c) III d) IV e) V C Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 106) Which of the alcohols listed below would you expect to react most rapidly with HBr, assuming the reaction pathway is predominantly SN1? a) CH3CH2CH2CH2CH2CH2OH b) (CH3CH2)2CH2CH2OH c) (CH3)2CHCH2CHOHCH3 d) CH3CH2CH2CH2CH2OH e) (CH3CH2)2C(CH3)OH E Topic: Alcohol Reaction Rates Section: 11.8 Difficulty Level: Easy 50 107) Which of the alcohols listed below would you expect to react most rapidly with PBr3? a) CH3CH2CH2CH2CH2CH2OH b) (CH3CH2)2CH(OH)CH2CH3 c) (CH3CH2)2CHOHCH3 d) (CH3CH2)3COH e) (CH3CH2)2C(CH3)OH A Topic: Alcohol Reaction Rates Section: 11.9 Difficulty Level: Easy 108) Which alcohol would undergo acid-catalyzed dehydration most rapidly? a) 3,3-dimethyl-1-butanol b) 2,2-dimethyl-1-butanol c) 3,3-dimethyl-2-butanol d) 2-methyl-2-butanol e) All would undergo dehydration equally rapidly. D Topic: Alcohol Reaction Rates Section: 11.8 Difficulty Level: Medium 109) Assuming the mechanistic pathway for each of the following reactions is predominately SN1, which of these alkyl halide syntheses is predicted to occur at the greatest rate? a) CH3CH2CH2CH2OH + HI b) (CH3)2CHCH2OH + HBr c) CH3CHOHCH2CH3 + HCl d) CH3CHOHCH2CH3 + HBr e) (CH3)3COH + HI E Topic: Alcohol Reaction Rates Section: 11.8 Difficulty Level: Easy 51 110) The following reaction, CH3CH2CH2CH2OH heat 3CH2CH2CH2Br + H2O HBr is probably: a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. B Topic: Reaction Mechanisms Section: 11.8 Difficulty Level: Easy 111) Which statement is true concerning the formation of alcohols by the hydroborationoxidation sequence? a) Overall, the process results in syn addition and anti-Markovnikov orientation. b) Overall, the process results in anti addition and anti-Markovnikov orientation. c) Overall, the process results in syn addition and Markovnikov orientation. d) Overall, the process results in anti addition and Markovnikov orientation. e) The stereochemistry and orientation are unpredictable. A Topic: Reaction Mechanisms Section: 11.4 Difficulty Level: Easy 112) What is the electrophilic species involved in the initial step of the reaction below? OH HgOAc Hg(OAc)2 THF, H2O a) +OH b) +HgOAc c) H3O+ d) THF 52 e) the THF/H2O complex B Topic: Reaction Mechanisms Section: 8.6 and 11.4 Difficulty Level: Medium 113) What is the nucleophilic species involved in the initial step of the reaction below? OH HgOAc Hg(OAc)2 THF, H2O a) -OH b) Hg(OAc)2 c) H2O d) cyclopentene e) the THF/H2O complex D Topic: Reaction Mechanisms Section: 8.6 and 11.4 Difficulty Level: Medium 114) The reaction between 1-pentanol and HBr to yield 1-bromopentanol is probably: a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. B Topic: Reaction Mechanisms Section: 11.8 Difficulty Level: Medium 115) The reaction between 2-methyl-2-pentanol and HBr to yield 2-bromo-2-methylpentane is probably: 53 a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. A Topic: Reaction Mechanisms Section: 11.8 Difficulty Level: Medium 116) The reaction between 2-methyl-2-pentanol and HBr to yield 2-methyl-2-pentene is probably: a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. C Topic: Reaction Mechanisms Section: 11.8 Difficulty Level: Medium 117) The reaction between 4-methyl-1-pentanol and HBr to yield 4-methyl-1-pentene is probably: a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. D Topic: Reaction Mechanisms Section: 11.8 Difficulty Level: Medium 118) The following reaction, 54 2 CH3CH2CH2CH2OH (CH3CH2CH2CH2)2O + H2O H2SO4 heat is probably: a) an SN1-type reaction involving the protonated alcohol as the substrate. b) an SN2-type reaction involving the protonated alcohol as the substrate. c) an E1-type reaction involving the protonated alcohol as the substrate. d) an E2-type reaction involving the protonated alcohol as the substrate. e) an epoxidation reaction. B Topic: Reaction Mechanisms Section: 11.11 Difficulty Level: Medium 119) Assuming an overall exothermic process, select the potential energy diagram that best represents the following reaction: OH H+ heat (-H2O) 55 a) I b) II c) III d) IV e) V B Topic: Alcohol Reactions, Mechanisms Section: 11.8 Difficulty Level: Medium 120) Which of the following could not be used to synthesize 2-bromopentane efficiently? a) 1-Pentene + HBr b) 2-Pentene + HBr c) 2-Pentanol + HBr d) 2-Pentanol + PBr3 e) All of these choices would afford good yields of 2-bromopentane. B 56 Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Easy 121) Which of the following could be used to synthesize 2-bromobutane? a) CH3CH2CHCH2 + Br2 (aq) b) CH3CH2CCH3 + HBr c) CH3CH2CCH + HBr d) CH3CH2CCH + Br2 e) More than one of these choices. B Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Medium 122) Which of the following could be used to synthesize 2-bromobutane? a) b) c) d) e) More than one of these choices. B Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Medium 123) Which of the following could be used to synthesize 2-iodobutane? a) CH3CH2CHCH2 + I2 (aq) b) CH3CH2CCH3 + HI 57 c) CH3CH2CCH + HI d) CH3CH2CCH + I2 e) None of these choices. B Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Medium 124) Which of the following could be used to synthesize 2-chlorobutane? a) CH3CH2CHCH2 + Cl2 (aq) b) CH3CH2CCH3 + HCl c) CH3CH2CCH + HCl d) CH3CH2CCH + Cl2 e) None of these choices. B Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Medium 125) Which of the following could be used to synthesize 2-chlorobutane? a) b) c) d) e) None of these choices. B Topic: Alcohol Reactions Section: 11.8 Difficulty Level: Medium 58 126) Which of the following could be used to synthesize 1-bromobutane efficiently? a) CH3CH2CH=CH2 + HBr b) CH3CH2CH2CH2OH + PBr3 c) CH3CH2CH2(OH)CH3 + HBr d) CH3CH2CH2CH2OH + Br2 e) None of these choices. B Topic: Alcohol Reactions Section: 11.9 Difficulty Level: Medium 127) Which of the following could be used to synthesize 1-bromopentane? a) CH3CH2CH2CH=CH2 + HBr b) CH3CH2CH2CH2CH2OH + PBr3 c) CH3CH2CH2CH2CH2OH + NaBr d) CH3CH2CH2CH2CH2OH + Br2 e) CH3CH2CH2CH=CH2 + Br2 B Topic: Alcohol Reactions Section: 11.9 Difficulty Level: Medium 128) The conversion of CH CH CH3 OH H3C CH3 to CH CH CH3 Br H3C CH3 is best achieved through use of which of these reagents in a low temperature reaction? a) Concd. HBr b) Br2 c) NaBr, H2SO4 d) PBr3 e) HBr, peroxide D 59 Topic: Alcohol Reactions Section: 11.9 Difficulty Level: Medium 129) The conversion of 3-methyl-1-octanol to 1-chloro-3-methyloctane is best achieved through use of which of these reagents? a) Concd. HCl b) SO2Cl2 c) NaCl, H2SO4 d) PCl3 e) POCl3 D Topic: Alcohol Reactions Section: 11.9 Difficulty Level: Medium 130) The reaction between 4-methyl-1-pentanol and PBr3 to yield 1-bromo-4-methylpentane is probably: a) an SN1-type reaction involving the protonated alkyl dibomophosphite of the alcohol as the substrate. b) an SN2-type reaction involving the protonated alkyl dibomophosphite of the alcohol as the substrate. c) an E1-type reaction involving the protonated alkyl dibomophosphite of the alcohol as the substrate. d) an E2-type reaction involving the protonated alkyl dibomophosphite of the alcohol as the substrate. e) an epoxidation reaction. B Topic: Reaction Mechanisms Section: 11.9 Difficulty Level: Medium 131) Which of the following reactions would serve as a synthesis of butyl bromide? a) CH3CH2CH2CH2OH + HBr reflux b) CH3CH2CH2CH2OH + PBr3 60 c) CH3CH2CH2CH2OH + NaBr reflux d) CH3CH2CH2CH2OH + Br2 e) Two of these choices. E Topic: Alcohol Reactions Section: 11.8 and 11.9 Difficulty Level: Medium 132) Which reagent(s) would transform propyl alcohol into propyl bromide? a) Concd. HBr and heat b) PBr3 c) NaBr/H2O and heat d) More than one of these choices. e) All of these choices. D Topic: Alcohol Reactions Section: 11.8 and 11.9 Difficulty Level: Medium 133) What is the product of the reaction of propyl alcohol with (CH3)3SiCl in the presence of a tertiary amine? a) CH3CH2CH2Si(CH3)3 b) (CH3)2CHSi(CH3)3 c) CH3CH2CH2OSi(CH3)3 d) (CH3)2CHOSi(CH3)3 e) (CH3CH2CH2)3SiOH C Topic: Alcohol Reactions Section: 11.11 Difficulty Level: Medium 134) What would be the major product of the following reaction sequence? 61 H H OH CH3 H H I CH3 I H H CH3 H I I CH3 H H OSO2I CH3 CH3SO2Cl base mesylate NaI ethanol ? I II III IV a) I b) II c) III d) IV e) An equimolar mixture of I and II. B Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 135) What would be the major product of the following reaction sequence? OH H CH3 H CH3SO2Cl base mesylate NaI ethanol ? H H CH3 I I H CH3 H H I CH3 I H H CH3 SO2I I II III IV a) I b) II c) III d) IV 62 e) An equimolar mixture of I and II. A Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 136) What would be the major product of the following reaction sequence? OH H CH3 H pCH3C6H4SO2Cl base tosylate NaI ethanol ? H H CH3 I I H CH3 H H I CH3 I H H CH3 SO2C6H4CH3 I II III IV a) I b) II c) III d) IV e) An equimolar mixture of I and II. B Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 137) What would be the major product of the following reaction sequence? 63 OH H CH3 H pCH3C6H4SO2Cl base tosylate NaBr ethanol ? H H CH3 Br Br H CH3 H H Br CH3 Br H H CH3 SO2C6H4CH3 I II III IV a) I b) II c) III d) IV e) An equimolar mixture of II and III. C Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 138) What would be the major product of the following reaction sequence? OH H CH3 H pCH3C6H4SO2Cl base tosylate NaOH ? H H CH3 OH OH H CH3 H H OH CH3 OH H H CH3 SO2C6H4CH3 I II III IV H2O a) I 64 b) II c) III d) IV e) An equimolar mixture of I and II. A Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 139) cis-3-Methylcyclopentanol is treated with CH3SO2Cl in the presence of a base. The product of the reaction then is allowed to react with KI in methanol. What is the final product? a) trans-1-Iodo-3-methylcyclopentane b) cis-1-Iodo-3-methylcyclopentane c) 1-Methylcyclopentene d) 2-Methylcyclopentene e) 3-Methylcyclopentene A Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 140) The major product of the following reaction would be: CH3SO2Cl base Product ? I II III C2H5 H OH CH3 CH3CO2- C2H5 H3CCO2 H CH3 C2H5 H O2CCH3 CH3 C2H5 H3CCO2 OSO2CH3 CH3 a) I b) II 65 c) III d) Equal amounts of I and II. e) None of these choices. A Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 141) trans-3-Methylcyclopentanol is treated with CH3SO2Cl in the presence of base. The product of this reaction is then heated with KI in methanol. What is the final product? a) trans-1-Iodo-3-methylcyclopentane b) cis-1-Iodo-3-methylcyclopentane c) 1-Methylcyclopentene d) 2-Methylcyclopentene e) 3-Methylcyclopentene B Topic: Alcohol Reactions Section: 11.10 Difficulty Level: Hard 142) Methanesulfonic acid, S O OH, O CH3 is treated, in turn, with PCl5 and (R)-2-butanol. Which of the following Fischer formulas is a stereochemically correct representation of the final product? 66 CH3 CH2CH3 MsO H H CH2CH3 MsO CH3 H CH2CH3 S CH3 O O H3C CH3 CH2CH3 S H O O H3C CH3 CH2CH3 S Cl O O H3C I II III IV V a) I b) II c) III d) IV e) V A Topic: Alcohol Reactions/Stereochemistry Section: 11.10 Difficulty Level: Hard 143) The major industrial process in use today for the production of methanol is the: a) hydration of ethyne. b) distillation of wood. c) hydrogenation of carbon dioxide. d) reduction of methanal. e) catalytic reduction of carbon monoxide. E Topic: General Section: 11.3 Difficulty Level: Easy 144) Today, most industrial ethanol is made in the U.S. by the: a) fermentation of grain. 67 b) hydrolysis of ethyl bromide. c) hydration of ethylene. d) reduction of acetaldehyde. e) hydration of acetylene. C Topic: General Section: 11.3 Difficulty Level: Easy 145) Which compound is a tosylate? CH3 S O O O CH3 Br S O O O CH2 CH3 S O O CH3 CH3 S CH3 O CH3 S O O CH3 CH3 O I II III IV V a) I b) II c) III d) IV e) V A Topic: General Section: 11.10 Difficulty Level: Easy 146) Which compound is a mesylate? 68 a) I b) II c) III d) IV e) V C Topic: General Section: 11.10 Difficulty Level: Easy 147) Which is the best way to prepare 3-methoxypentane via the Williamson method? a) CH3OH + CH3CH2CHOHCH2CH3 + H2SO4, 140C b) CH3OH + (CH3)2CHCH2CH2OH + H2SO4, 140C c) CH3ONa + (CH3CH2)2CHBr d) CH3I + (CH3CH2)2CHONa e) CH3I + (CH3)2CHCH2CH2ONa D Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 148) Which method would provide the best synthesis of ethyl isopropyl ether? a) (CH3)2CHONa + CH3CH2Br b) CH3CH2ONa + (CH3)2CHBr c) CH3CH2OH + (CH3)2CHOH H2SO4, 140 oC 69 d) CH3CH2OH + (CH3)2CHOH H2SO4, 180 oC e) CH3CH2ONa + (CH3)2CHOH A Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 149) Which is the best method for the synthesis of tert-butyl methyl ether? a) CH3ONa + (CH3)3CBr b) (CH3)3CONa + CH3I c) d) (CH3)3CONa + CH3OCH3 e) CH3ONa + (CH3)3COH B Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 150) Which is the best method to prepare 2-ethoxy-5-methylhexane? a) C2H5ONa + (CH3)2CHCH2CH2Br b) C2H5ONa + (CH3)2CHCH2CH2CH2CH2Br c) C2H5ONa + (CH3)2CHCH2CH2CHBrCH3 d) C2H5Br + (CH3)2CHCH2CH2CH(CH3)ONa e) D Topic: Ether Synthesis Section: 11.11 Difficulty Level: Hard 151) When 3-methyl-2-pentene is treated with mercuric acetate, Hg(O2CCH3)2, in a THF-ethanol mixture and the resulting product reacted with NaBH4 in basic solution, the principal product formed is which of these? 70 a) 3-methyl-3-pentanol b) 3-ethoxy-3-methylpentane c) 3-methyl-2-pentanol d) 2-ethoxy-3-methylpentane e) 1-ethoxy-3-methylpentane B Topic: Ether Synthesis Section: 11.11 Difficulty Level: Hard 152) When 3-methyl-2-pentene is treated with mercuric acetate, Hg(O2CCH3)2, in a THF-t-butyl alcohol mixture and the resulting product reacted with NaBH4 in basic solution, the principal product formed is which of these? a) 3-methyl-3-pentanol b) 3-t-butoxy-3-methylpentane c) 3-methyl-2-pentanol d) 2- t-butoxy -3-methylpentane e) 1- t-butoxy -3-methylpentane B Topic: Ether Synthesis Section: 11.11 Difficulty Level: Hard 153) The product of the following reaction 71 1. Hg(OAc)2, (CH3)3COH, THF 2. NaBH4, -OH is: I II IV O O III OH O V O a) I b) II c) III d) IV e) V A Topic: Ether Synthesis Section: 11.11 Difficulty Level: Hard 154) Which of these ethers is most resistant to peroxide formation on exposure to atmospheric oxygen? a) CH3OCH2CH3 b) CH3CH2OCH2CH3 c) (CH3)2CHOCH(CH3)2 d) (CH3)2CHOCH2CH3 e) CH3OC(CH3)3 E Topic: Ether Reaction Rates 72 Section: 11.3 Difficulty Level: Easy 155) Which of these ethers is least likely to undergo significant cleavage by hot aqueous H2SO4? I CH3OCH3 II CH3OCH(CH3)2 III (CH3)2CHOCH(CH3)2 IV V (CH3)3COC(CH3)3 O a) I b) II c) III d) IV e) V A Topic: Ether Reaction Rates Section: 11.12 Difficulty Level: Easy 156) Which of the reagents listed below would serve as the basis for a simple chemical test to distinguish between O and ? a) AgNO3 in alcohol b) NaOH in H2O c) Br2 in CCl4 d) Cold conc. H2SO4 e) KMnO4 in H2O D Topic: Chemical Tests Section: 11.12 73 Difficulty Level: Medium 157) Which of the reagents listed below would serve as the basis for a simple chemical test to distinguish between O and Br ? a) AgNO3 in C2H5OH b) Dilute HCl c) Br2 in CCl4 d) NaOH in H2O e) KMnO4 in H2O A Topic: Chemical Tests Section: 11.12 Difficulty Level: Medium 158) The product(s) of the following reaction O excess HBr heat is/are: O OH and Br OH and Br Br Br O Br I II III IV a) I b) II c) III d) IV e) None of these choices. 74 C Topic: Ether Reactions Section: 11.12A Difficulty Level: Medium 159) The product(s) of the following reaction O excess HI heat is/are: O OH and I OH and I I I O I I II III IV a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 160) The product(s) of the following reaction 75 O 1 equiv. HI heat is/are: O OH and I OH I O I I II III IV a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 161) The product(s) of the following reaction 76 O 1 equiv. HI heat is/are: O OH I O I I II III IV OH I a) I b) II c) III d) IV e) None of these choices. B Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 162) The product(s) of the following reaction 77 a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 163) The product(s) of the following reaction 78 a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 164) The product(s) of the following reaction O 1 equiv. HI heat is/are: O OH I O I I II III IV OH I 79 a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 165) The product(s) of the following reaction 1 equiv. HI heat is: O OH I II III IV I O HO O OH I a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 166) The product(s) of the following reaction 80 1 equiv. HBr heat is: O OH I II III IV Br O HO O OH Br a) I b) II c) III d) IV e) None of these choices. C Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 167) What would be the major product(s) of the following reaction C6H5CH2OCH3 ? heat Concd. HBr (xs) a) C6H5Br + CH3OH b) C6H5CH2Br + CH3Br c) C6H5CH2OH + CH3Br d) C6H5CH2Br + CH3OH e) C6H5CH2CH2Br B Topic: Ether Reactions Section: 11.12 81 Difficulty Level: Medium 168) What would be the major product(s) of the following reaction O 1 equiv. HBr(conc) ? heat a) C6H5Br + CH3OH b) C6H5CH2Br + CH3Br c) C6H5CH2OH + CH3Br d) C6H5CH2Br + CH3OH e) C6H5CH2CH2Br D Topic: Ether Reactions Section: 11.12 Difficulty Level: Medium 169) Heating 2-ethoxyhexane with excess concentrated HBr would produce: a) CH3CH2OCH2CH2CH2CH2CH2CH2Br b) BrCH2CH2OCH2CH2CH2CH2CH2CH3 c) CH3CH2OH and CH3CH2CH2CH2CHBrCH3 d) CH3CH2Br and CH3CH2CH(OH)CH2CH2CH3 e) CH3CH2Br and CH3CH2CH2CH2CHBrCH3 E Topic: Ether Reactions Section: 11.14 Difficulty Level: Medium 170) Heating 2-ethoxyhexane with one equivalent of concentrated HBr would produce: a) CH3CH2OCH2CH2CH2CH2CH2CH2Br b) BrCH2CH2OCH2CH2CH2CH2CH2CH3 c) CH3CH2OH and CH3CH2CH2CH2CHBrCH3 d) CH3CH2Br and CH3CH2CH(OH)CH2CH2CH3 e) CH3CH2Br and CH3CH2CH2CH2CH(OH)CH3 82 E Topic: Ether Reactions Section: 11.14 Difficulty Level: Medium 171) Heating 2-ethoxyhexane with one equivalent of concentrated HI would produce: a) CH3CH2OCH2CH2CH2CH2CH2CH2I b) ICH2CH2OCH2CH2CH2CH2CH2CH3 c) CH3CH2OH and CH3CH2CH2CH2CHICH3 d) CH3CH2I and CH3CH2CH(OH)CH2CH2CH3 e) CH3CH2I and CH3CH2CH2CH2CH(OH)CH3 E Topic: Ether Reactions Section: 11.14 Difficulty Level: Medium 172) Select the potential energy diagram that best represents the following reaction: OCH3 HI I + CH3OH 83 a) I b) II c) III d) IV e) V E Topic: Ether Reactions, Mechanisms Section: 11.12 Difficulty Level: Medium 173) Assuming an overall exothermic process, select the potential energy diagram that best represents the following reaction: O HI OH + CH3I 84 a) I b) II c) III d) IV e) V A Topic: Ether Reactions, Mechanisms Section: 11.12 Difficulty Level: Medium 174) If cis-2-butene is treated with meta-chloroperbenzoic acid what is the final product? O H3C CH3 H H O H3C H H CH3 O H3C H H3C H O H3CH2C H H H I II III IV a) I b) II c) III 85 d) IV e) None of these choices. A Topic: Epoxide Synthesis Section: 11.13 Difficulty Level: Medium 175) If trans-2-butene is treated with meta-chloroperbenzoic acid what is the final product? O H3C CH3 H H O H3C H H CH3 O H3C H H3C H O H3CH2C H H H I II III IV a) I b) II c) III d) IV e) None of these choices. B Topic: Epoxide Synthesis Section: 11.13 Difficulty Level: Medium 176) If (Z)-2-pentene is treated with meta-chloroperbenzoic acid what is the final product? O H3CH2C CH3 H H O H3CH2C H H CH3 O H3CH2C H H3C H I II III a) I b) II c) III d) All of these choices. e) None of these choices. A Topic: Epoxide Synthesis Section: 11.13 86 Difficulty Level: Medium 177) If (e)-2-pentene is treated with meta-chloroperbenzoic acid what is the final product? O H3CH2C CH3 H H O H3CH2C H H CH3 O H3CH2C H H3C H I II III a) I b) II c) III d) All of these choices. e) None of these choices. B Topic: Epoxide Synthesis Section: 11.13 Difficulty Level: Medium 178) If trans-2-bromocyclohexanol is treated with sodium hydroxide what is the major product? a) I b) II c) III d) IV e) None of these choices. C Topic: Epoxide Synthesis Section: 11.11 Difficulty Level: Medium 179) If trans-2-bromocyclopentanol is treated with sodium hydroxide what is the major product? 87 O OH OH Br I II III IV a) I b) II c) III d) IV e) None of these choices. B Topic: Epoxide Synthesis Section: 11.11 Difficulty Level: Medium 180) The major product expected from the sequential reaction of cyclopentene with Br2/H2O, followed by sodium hydroxide is: a) I b) II c) III d) IV e) None of these choices. B Topic: Epoxide Synthesis Section: 11.11 Difficulty Level: Medium 181) The major product expected from the sequential reaction of cyclopentene with Br2/H2O, 88 followed by sodium hydroxide is: a) I b) II c) III d) IV e) None of these choices. C Topic: Epoxide Synthesis Section: 11.11 Difficulty Level: Medium 182) Select the structure of the major product formed in the following reaction. CH3CH O CH2 HA H218O ? a) CH3CH2CH218OH b) CH3CHCH3 18OH c) CH3CHCH2OH 18OH d) CH3CH OH CH2 18OH e) CH3CHCH218OH 18OH C Topic: Epoxide Reactions, Isotope Labeling Section: 11.14 Difficulty Level: Medium 89 183) Epoxidation followed by reaction with aqueous base converts cyclopentene into which of these? I II III IV H OH H OH H OH OH H H OH H OH OH H a) I b) II c) III d) IV e) Equal amounts of III and IV E Topic: Epoxide Reactions Section: 11.14 Difficulty Level: Medium 184) What would be the major product of the following reaction sequence? O ? CH3OCH3OH OH OCH3 OCH3 OCH3 OCH3 OH OH OH I II III IV H3O+ a) I b) II c) III d) IV e) Equal amounts of II and IV A Topic: Epoxide Reactions Section: 11.14 90 Difficulty Level: Medium 185) What would be the major product of the following reaction sequence? O H+ ? CH3OH OH OCH3 OCH3 OCH3 OCH3 OH OH OH I II III IV a) I b) II c) III d) IV e) Equal amounts of II and IV C Topic: Epoxide Reactions Section: 11.14 Difficulty Level: Medium 186) Which compound (or compounds) would be produced when trans-2-butene is treated first with a peroxy acid to form an epoxide, and then the epoxide is subjected to acid-catalyzed hydrolysis? C C HO CH3 H CH3 H OH I C C HO H HO CH3 CH3 H II C C H OH H3C H CH3 OH III a) An equimolar mixture of I and II b) An equimolar mixture of II and III c) I alone 91 d) II alone e) III alone E Topic: Epoxide Reactions Section: 11.15 Difficulty Level: Hard 187) Which compound (or compounds) would be produced when cis-2-butene is treated first with a peroxy acid to form an epoxide, and then the epoxide is subjected to acid-catalyzed hydrolysis? C C HO CH3 H CH3 H OH I C C HO H HO CH3 CH3 H II C C H OH H3C H CH3 OH III a) An equimolar mixture of I and II b) An equimolar mixture of II and III c) I alone d) II alone e) III alone A Topic: Epoxide Reactions Section: 11.15 Difficulty Level: Hard 188) What would be the final product? H3CC CH2 CH3 RCOOH O product CH3OH, HA final product a) (CH3)2CHCH2OCH3 b) (CH3)2CCH3 OCH3 92 c) (CH3)2CCH2OH OCH3 d) (CH3)2CCH2OCH3 OH e) (CH3)2CCH2OCH3 OCH3 C Topic: Epoxide Synthesis /Reactions Section: 11.13 and 11.14 Difficulty Level: Medium 189) What would be the major product of the following reaction sequence? ? I II III IV RCOOOH NH3 O OH OH OH NH2 NH2 OH a) I b) II c) III d) IV e) Equal amounts of II and IV C Topic: Epoxide Synthesis/Reactions Section: 11.13 and 11.14 Difficulty Level: Medium 190) cis-3-Hexene is treated with meta-chloroperbenzoic acid and the product is then subjected to acid-catalyzed hydrolysis. What is the final product? 93 CH2CH3 CH2CH3 H H H OH I II CH2CH3 CH2CH3 H H HO H CH2CH3 CH2CH3 H OH HO H III IV CH2CH3 CH2CH3 HO H H OH V CH2CH3 CH2CH3 H OH H OH a) equal amounts I and II b) equal amounts I, II and V c) equal amounts III, IV and V d) equal amounts III and IV e) Only V D Topic: Epoxide Synthesis/Reactions Section: 11.13 and 11.14 Difficulty Level: Hard 191) trans-3-Hexene is treated with meta-chloroperbenzoic acid and the product is then subjected to acid-catalyzed hydrolysis. What is the final product? CH2CH3 CH2CH3 H H H OH I II CH2CH3 CH2CH3 H H HO H CH2CH3 CH2CH3 H OH HO H III IV CH2CH3 CH2CH3 HO H H OH V CH2CH3 CH2CH3 H OH H OH a) equal amounts I and II b) equal amounts I, II and V c) equal amounts III, IV and V d) equal amounts, III and IV e) Only V E Topic: Epoxide Synthesis/Reactions Section: 11.13 and 11.14 Difficulty Level: Hard 192) What is the correct IUPAC name for the following compound? 94 O O O O O a) 15-crown-5 b) 15-crown-4 c) 5-crown-15 d) 15-crown-15 e) Cyclopentadecane pentaether A Topic: Ether Nomenclature Section: 11.16 Difficulty Level: Hard 193) A correct IUPAC name for the following compound is? a) 6-crown-2 b) 6-crown-3 c) 3-crown-6 d) 2-crown-6 e) oxabenzene B Topic: Ether Nomenclature Section: 11.16 Difficulty Level: Hard 194) What is the correct IUPAC name for the following compound? 95 O O O O a) 12-crown-5 b) 12-crown-4 c) 4-crown-12 d) 12-crown-12 e) Cyclododecane tetraether B Topic: Ether Nomenclature Section: 11.16 Difficulty Level: Hard Question type: Molecular Drawing 195) Draw structures for all possible ethers having the formula C4H10O C4H10O: ethers O O O Topic: Isomers Section: 11.1 Difficulty Level: Easy 196) Draw all of the ethers corresponding to the formula C5H12O, including stereoisomers. 96 O O O O O H O H O Topic: Isomers Section: 11.1 Difficulty Level: Medium 197) Draw all of the primary alcohols corresponding to the formula C5H12O, including stereoisomers. OH OH OH OH H OH H Topic: Isomers Section: 11.1 Difficulty Level: Medium 198) Draw all of the enantiomeric forms corresponding to the formula C5H12O. OH OH H H HO H HO H HO H HO H Topic: Isomers Section: 11.1 Difficulty Level: Medium 97 199) Draw all the enantiomeric forms of ethers with the formula C5H12O. O H O H Topic: Isomers Section: 11.1 Difficulty Level: Medium Question type: Essay 200) Give the correct IUPAC name corresponding to the following structure: OH (R)-3-ethyl-2-methyl-3-hexanol Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 201) Give the correct IUPAC name corresponding to the following structure: CH3 OH Br H (1R,2R)-2-bromo-1-methylcyclopentanol Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 202) Give the correct IUPAC name corresponding to the following structure: 98 OH 6-cyclohexyl-2,7-dimethyl-3-octanol Topic: Nomenclature Section: 11.1 Difficulty Level: Hard 203) Give the correct IUPAC name corresponding to the following structure: O O 1,3-diethoxyhexane Topic: Nomenclature Section: 11.1 Difficulty Level: Hard Question type: fill-in-the-blank 204) Long-term storage of ethers can be dangerous, because most ethers react slowly with oxygen by a radical process called ___. This process forms peroxides and hydroperoxides which are dangerously ___. autooxidation, explosive Topic: Ether Reactivity Section: 11.3 Difficulty Level: Easy 205) We have learned three different methods to convert alkenes into alcohols. a) The method that takes place with Markovnikov regioselectivity but is prone to rearrangement is ___. b) The method that takes place with Markovnikov regioselectivity and does not lead to rearrangement is ___. c) The method that takes place with anti-Markovnikov regioselectivity and syn stereoselectivity is ___. 99 a) acid-catalyzed hydration; b) oxymercuration-demercuration; c) hydroborationoxidation Topic: General, Alcohol Synthesis Section: 11.4 Difficulty Level: Easy Question type: Essay 206) What reaction is needed to accomplish the following transformation: H3O+ OH 0xymercuration/demurcuration Topic: Alcohol Synthesis/Mechanism Section: 11.4 Difficulty Level: Medium 207) Propose a mechanism for the following transformation: H3O+ OH Topic: Alcohol Synthesis/Mechanism Section: 11.4 Difficulty Level: Hard 100 208) Draw the structures of all the products formed when (3R)-3-methylcyclopentene is subjected to the hydroboration-oxidation sequence. The alkene is symmetrically substituted: thus, Markovnikov rule does not apply. Hence, all four products shown below are likely to be formed in roughly equal proportions. H3C H (3R)-3-methylcyclopentene hydroboration-oxidation H3C H H3C H H3C H H3C H OH OH OH OH + + + Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 209) Draw the products formed from the oxymercuration-demercuration of 3- methylcyclopentene. OH OH and Topic: Alcohol Synthesis Section: 11.4 Difficulty Level: Hard 210) Reactions of alcohols can be broadly categorized into three types. a) Those that utilize the hydroxyl oxygen as ___. b) Those that utilize the hydroxyl proton as ___. c) Those that convert the hydroxyl group into ___. a) nucleophile or base; b) an acid; c) a leaving group 101 Topic: General, Alcohol Reactions Section: 11.5 Difficulty Level: Easy 211) Finish the following acid-base reaction and predict if it will proceed in the forward direction: OH + NaNH2 OH + NaNH2 ONa + NH3 pKa = 18 pKa = 35 Yes Topic: Alcohol as Acids Section: 11.6 Difficulty Level: Easy 212) Finish the following acid-base reaction and predict if it will proceed in the forward direction: OH + Na+ C CH OH + Na+ C CH ONa + HC CH pKa = 18 pKa = 25 Yes Topic: Alcohol as Acids Section: 11.6 Difficulty Level: Easy 213) Finish the following acid-base reaction and predict if it will proceed in the forward direction: 102 OH + CH3COONa OH + CH3COONa ONa + CH3COOH pKa = 18 pKa = 5 No Topic: Alcohol as Acids Section: 11.6 Difficulty Level: Easy Question type: fill-in-the-blank 214) Three-membered ring cyclic ethers are called ___ or ___. oxiranes; epoxides Topic: General Section: 11.13 Difficulty Level: Easy 215) The SN2 reaction between an alkoxide and an alkyl halide is commonly referred to as the ___ synthesis. Williamson Topic: General Section: 11.11 Difficulty Level: Easy 216) Stereochemically speaking, conversion of an alcohol into a tosylate occurs with ___ of configuration. retention Topic: General Section: 11.10 Difficulty Level: Easy 103 217) Generally the process of hydroboration-oxidation of an unsymmetrical substituted alkene proceeds with ___ orientation. anti-Markovnikov Topic: General Section: 11.10 Difficulty Level: Easy 218) When an alcohol in which the OH is attached to a stereogenic carbon reacts with thionyl chloride (SOCl2) in the presence of a 3° amine, the resulting alkyl chloride is produced with ___ of configuration. inversion Topic: General Section: 11.9 Difficulty Level: Medium 219) A reaction which leads to a product of particular stereoisomeric outcome, depending on the stereochemistry of the reactant, is termed ___. stereospecific Topic: General Section: 11.13 Difficulty Level: Medium 220) The development of a procedure called ___ has made the use of nonpolar solvents possible in reactions involving polar reagents. phase transfer catalysis Topic: General Section: 11.16 Difficulty Level: Medium 221) The relationship between a crown ether and the ion it transports is known as the ___ relationship. 104 guest-host Topic: General Section: 11.16 Difficulty Level: Medium 222) A compound or ion that prefers a nonpolar environment to an aqueous one is said to be ___. lipophilic Topic: General Section: 11.16 Difficulty Level: Medium Question type: Essay 223) Complete the following reaction sequence, giving structures for compounds C and D: OH DMF CH3SO2Cl C CH3OH D Na2CO3 C = OMs D = = OCH3 Topic: Multistep Reactions Section: 11.10 Difficulty Level: Medium 224) Supply the missing reagents A and B. CH3 OH CH3 OTs A B CH3 CN Topic: Multistep Reactions 105 Section: 11.10 Difficulty Level: Medium 225) Supply the missing structures A and B. Br2 H2O KOH Topic: Multistep Reactions Section: 11.10 Difficulty Level: Medium 226) Supply the missing reagents A and B. Topic: Multistep Reactions Section: 11.10 Difficulty Level: Medium 227) Which is the most efficient way to prepare isopropyl methyl ether via the Williamson method? CH3I + (CH3)2CHONa Topic: Ether Synthesis Section: 11.11B 106 Difficulty Level: Easy 228) Propose a mechanism for the following transformation: HO Br -OH O O Br -OH H O Br O Topic: Ether Synthesis/Mechanism Section: 11.11 Difficulty Level: Medium 229) Predict the product of the following reaction: HO Br -OH O Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 230) Predict the product of the following reaction: HO Br -OH 107 O Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 231) Propose a mechanism for the following transformation: HO Br -OH O O -OH O H Br O Br Topic: Ether Synthesis/Mechanism Section: 11.11 Difficulty Level: Medium 232) Propose a mechanism for the following transformation: HO Br -OH O O Br -OH H O Br O Topic: Ether Synthesis/Mechanism Section: 11.11 108 Difficulty Level: Medium 233) Propose a mechanism for the following transformation: OH Br OH O O Br H OH O Br O Topic: Ether Synthesis/Mechanism Section: 11.11 Difficulty Level: Medium 234) Which is the best method to prepare ethoxycyclopentane via the Williamson method? ONa I O + Topic: Ether Synthesis Section: 11.11 Difficulty Level: Medium 235) Propose a mechanism for the following transformation: O 1 equiv. HI heat I + CH3OH 109 O H I O H CH2 -CH3OH II Topic: Ether Synthesis/Mechanism Section: 11.11 Difficulty Level: Medium 236) Complete the following reaction sequence, giving structures for compounds A and B: A = ONa B = = OCH3 Topic: Multistep Reactions Section: 11.6 and 11.11 Difficulty Level: Medium 237) Complete the following reaction sequence, giving structures for compounds A, B, C and D: Topic: Multistep Reactions 110 Section: 11.6 and 11.11 Difficulty Level: Medium 238) Supply the missing reagents A and B. A B OC(CH3)3 A = Hg(OAc)2, THF, HOC(CH3)3 B = NaBH4 Topic: Multistep Reactions Section: 11.11 Difficulty Level: Medium 239) Provide a reasonable synthetic strategy for the synthesis of trans-1,2-cyclohexanediol from bromocyclohexane Br OH CH3ONa m-CPBA H3O+ CH3OH O OH + enantiomer Topic: Epoxide Synthesis and Reactions Section: 11.13 and 11.14 Difficulty Level: Hard 240) Provide a reasonable synthetic strategy for the synthesis of trans-2-methoxycyclopentanol from bromocyclopentane CH3ONa CH3OH m-CPBA Br O CH3ONa CH3OH OH OCH3 + enantiomer Topic: Epoxide Synthesis and Reactions 111 Section: 11.13 and 11.14 Difficulty Level: Hard 241) Propose a mechanism for the following transformation: Topic: Epoxide Synthesis/Mechanism Section: 11.11 Difficulty Level: Medium 242) Propose a structure for the compound with the following formula that is consistent with the IR and 1H NMR data shown: C3H8O IR – 3350cm-1, strong, broad 6H doublet at 1.3 , J = 7 Hz 1H singlet at 2.2 , (exchangeable) 1H septet at 4.0 , J = 7 Hz OH Topic: Spectroscopy of Alcohols Section: 2.16 and 11.1 Difficulty Level: Medium 243) Propose a structure for the compound with the following formula that is consistent with the 112 IR and 1H NMR data shown: C7H8O IR – 3325cm-1, strong, broad 1H broad singlet at 2.3 (exchangeable) 2H singlet at 4.6 5H multiplet at 7.3 OH Topic: Spectroscopy of Alcohols Section: 2.16 and 11.1 Difficulty Level: Medium 244) A compound has the formula C6H14O. The 13C and 1H NMR spectral data for this compound are: 13 C NMR Broadband decoupled 13C NMR: 29.7, 29.8, 46.4, 60.0 δ DEPT-90: no peaks DEPT-135: positive peak at 29.8 δ; negative peaks at 29.7, 60.0 δ 1 H NMR 0.91 δ, singlet (9H) 1.53 δ, triplet (2H) J = 7.3Hz 2.13 δ, broad singlet (exchangeable, 1H) 3.70 δ, triplet (2H) J = 7.3Hz OH Topic: Spectroscopy of Alcohols Section: 2.16 and 11.1 Difficulty Level: Hard 245) Propose a structure for the compound with the following formula that is consistent with the IR and 1H NMR data shown: 113 C4H10O IR – 1125cm-1, strong, broad 6H triplet at 1.2 , J = 7 Hz 4H quartet at 3.7 , J = 7 Hz O Topic: Spectroscopy of Ethers Section: 2.16 and 11.1 Difficulty Level: Medium 246) Propose a structure for the compound with the following formula that is consistent with the IR and 1H NMR data shown: C6H14O IR – 1120cm-1, strong, broad 6H triplet at 0.9 4H sextet at 1.5 4H triplet at 3.4 O Topic: Spectroscopy of Ethers Section: 2.16 and 11.1 Difficulty Level: Medium 247) Propose a structure for the compound with the following formula that is consistent with the IR and 1H NMR data shown: C6H14O IR – 1120cm-1, strong, broad 3H triplet at 1.1 9H singlet at 1.15 2H quartet at 3.45 114 O Topic: Spectroscopy of Ethers Section: 9.2, 9.11, and 11.1 Difficulty Level: Hard 248) A compound has the formula C5H10, with the 13C and 1H NMR spectral data shown. Propose structure that fits this data. 13 C NMR Broadband decoupled 13C NMR: 23.5, 26.7, 68.9 δ DEPT-90: no peaks DEPT-135: no positive peaks; negative peaks at 23.5, 26.7, 68.9 δ 1 H NMR 1.7 δ, multiplet (6H) 3.72 δ, triplet (4H) J = 7Hz O Topic: Multistep Synthetic Strategy Section: 8.13 and 11.4 Difficulty Level: Hard 249) Provide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from methylcyclopentane: 115 CH3 OH Br H CH3 Br Br2 h CH3ONa CH3OH heat CH3 OH Br H + Br 2, H2O 2-bromo-1-methylcyclopentanol (1R,2R) (1S,2S) CH3 CH3 Topic: Multistep Synthetic Strategy Section: 8.13 and 11.4 Difficulty Level: Hard 250) Provide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from methylenecyclopentane: HBr Br NaOCH3 HOCH3 OH Br H + enantiomer Br2 H2O Topic: Multistep Synthetic Strategy Section: 8.15 and 11.4 Difficulty Level: Hard 251) Complete the following reaction sequence, giving structural details of all key intermediates: i) Li, NH3 ii) KMnO4 ,OH, H2O iii) H3O+ ? 116 Li NH3 i) KMnO4 ,OH, H2O ii) H3O+ OH OH H H H H HO HO + (2S,3S)-pentane-2,3-diol (2R,3R)-pentane-2,3-diol Topic: Multistep Reactions Section: 8.15 and 11.4 Difficulty Level: Hard 252) Complete the following reaction sequence, giving structural details of all key intermediates: i) P-2, H2 ii) KMnO4 ,OH, H2O iii) H3O+ ? P-2, H2 i) KMnO4 ,OH, H2O ii) H3O+ OH H H HO H OH HO H + (2S,3R)-pentane-2,3-diol (2R,3S)-pentane-2,3-diol Topic: Multistep Reactions Section: 8.15 and 11.4 Difficulty Level: Hard 253) Complete the following reaction sequence, giving structural details of all key intermediates: i) Lindlar catalyst, H2 ii) KMnO4 ,OH, H2O iii) H3O+ ? 117 Lindlar catalyst H2 i) KMnO4 ,OH, H2O ii) H3O+ OH H H HO H OH HO H + (2S,3R)-pentane-2,3-diol (2R,3S)-pentane-2,3-diol Topic: Multistep Reactions Section: 8.15 and 11.4 Difficulty Level: Hard 254) Complete the following reaction sequence, giving structural details of all key intermediates: i) H2, Lindlar catalyst ii) KMnO4 ,OH, H2O iii) H3O+ ? i) KMnO4 ,OH, H2O ii) H3O+ H H2 Lindlar catalyst Topic: Multistep Reactions OH H HO meso Section: 8.15 and 11.4 Difficulty Level: Hard 255) Complete the following reaction sequence, giving structural details of all key intermediates: i) H2, P-2 ii) KMnO4 ,OH, H2O iii) H3O+ ? 118 i) KMnO4 ,OH, H2O ii) H3O+ OH H H HO meso H2, P-2 Topic: Multistep Reactions Section: 11.13 and 11.14 Difficulty Level: Hard 256) Complete the following reaction sequence, giving structural details of all key intermediates: i) Li, NH3 ii) m-CPBA iii) H3O+, H2O ? Li NH3 H OH HO H OH H H HO + i) m-CPBA ii) H3O+, H2O (2R,3S)-pentane-2,3-diol (2S,3R)-pentane-2,3-diol Topic: Multistep Reactions Section: 11.13 and 11.14 Difficulty Level: Hard [Show More]
Last updated: 11 months ago
Preview 1 out of 118 pages

Reviews( 0 )
Recommended For You
Chemistry> TEST BANK > Structure and Nomenclature. Chemistry TEST BANK. Chapter Eleven. All Answers highlighted. Contains 256 Questions and Answers. (All)

Structure and Nomenclature. Chemistry TEST BANK. Chapter Eleven. All Answers highlighted. Contains 256 Questions and Answers.
Chapter Eleven MULTIPLE CHOICE QUESTIONS Topic: Structure and Nomenclature Section: 5.3 and 11.1 Difficulty Level: Hard 1. What is the relationship between alcohols I and II? CH3 OH CH3 HO I...
By QuizMaster , Uploaded: Apr 07, 2021
$7.5
Financial Accounting> TEST BANK > CHAPTER 1 ACCOUNTING IN ACTION: Test Bank for Accounting Principles, Eleventh Edition. This document/TEST BANK Contains 256 Questions With Answers, Worked Solutions and Essay Explanations (All)
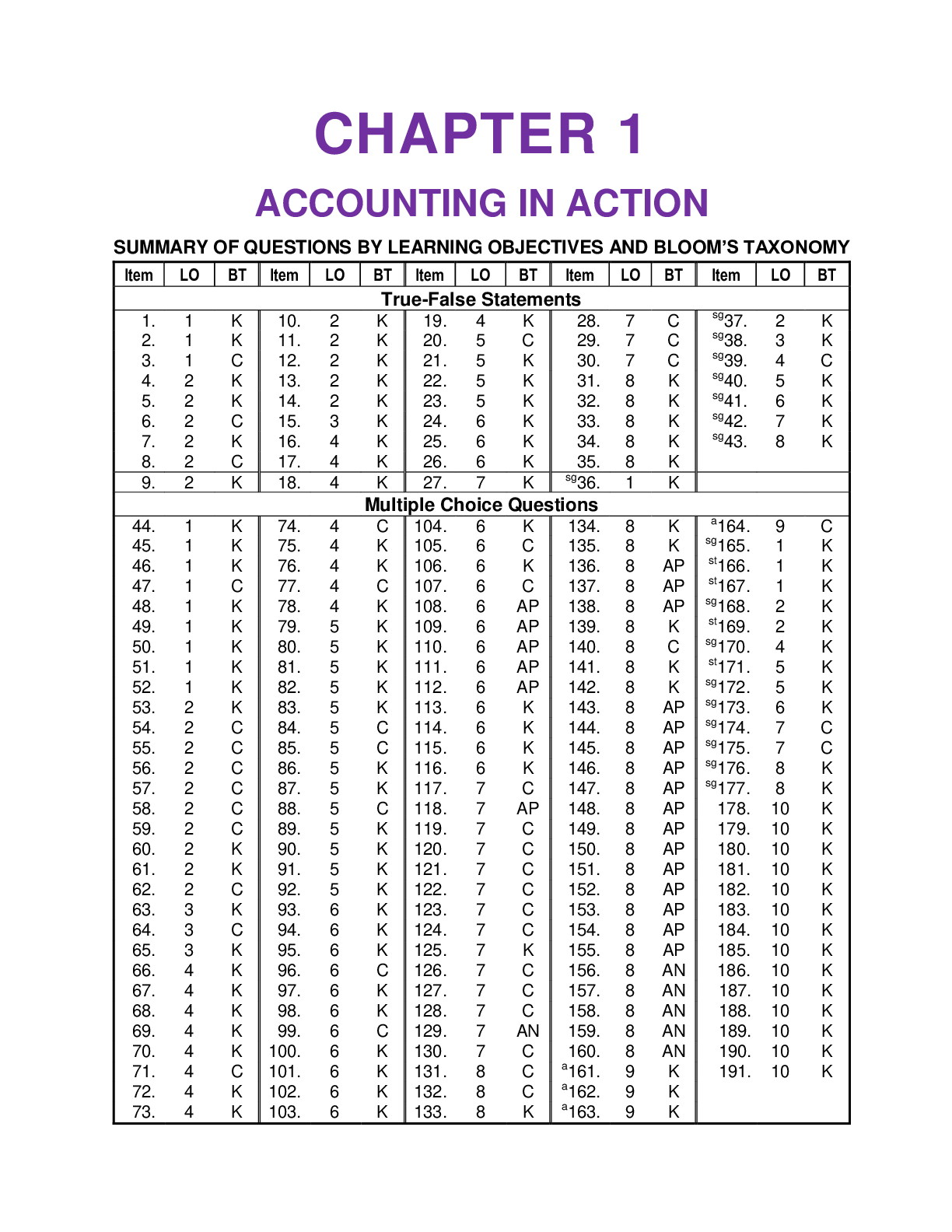
CHAPTER 1 ACCOUNTING IN ACTION: Test Bank for Accounting Principles, Eleventh Edition. This document/TEST BANK Contains 256 Questions With Answers, Worked Solutions and Essay Explanations
CHAPTER 1 ACCOUNTING IN ACTION SUMMARY OF QUESTIONS BY LEARNING OBJECTIVES AND BLOOM’S TAXONOMY sg This question also appears in the Study Guide. st This question also appears in a self-test a...
By QuizMaster , Uploaded: Jan 19, 2021
$6.5
Human Resource Management> TEST BANK > A Framework for Human Resource Management 7e Gary Dessler (Test Bank) (All)
.png)
A Framework for Human Resource Management 7e Gary Dessler (Test Bank)
This is the eBook of the printed book and may not include any media, website access codes, or print supplements that may come packaged with the bound book. A Framework for Human Resource Management pr...
By eBookSmTb , Uploaded: Mar 10, 2023
$25
Accounting> TEST BANK > Payroll Accounting 2023 9th Edition By Jeanette Landin, Paulette Schirmer (Test Bank, 100% Verified Original, A+ Grade) (All)

Payroll Accounting 2023 9th Edition By Jeanette Landin, Paulette Schirmer (Test Bank, 100% Verified Original, A+ Grade)
Payroll Accounting 2023, 9e Jeanette Landin, Paulette Schirmer (Test Bank, 100% Verified Original, A+ Grade) Payroll Accounting 2023, 9e Jeanette Landin, Paulette Schirmer (Test Bank, 100% Verified O...
By eBookSmTb , Uploaded: Oct 04, 2023
$25
Accounting> TEST BANK > McGraw Hills Essentials Of Federal Taxation 2024 15th Edition By Spilker, Ayers, Robinson, Outslay, Worsham, Barrick, Weaver (Test Bank, 100% Verified Original, A+ Grade) (All)

McGraw Hills Essentials Of Federal Taxation 2024 15th Edition By Spilker, Ayers, Robinson, Outslay, Worsham, Barrick, Weaver (Test Bank, 100% Verified Original, A+ Grade)
McGraw Hills Essentials Of Federal Taxation 2024, 15e Spilker, Ayers, Robinson, Outslay, Worsham, Barrick, Weaver (Test Bank, 100% Verified Original, A+ Grade) McGraw Hills Essentials Of Federal Taxa...
By eBookSmTb , Uploaded: Oct 04, 2023
$25
Marketing> TEST BANK > Marketing Strategy A Decision Focused Approach 8th Edition By John Mullins, Orville Walker (Test Bank, 100% Verified Original, A+ Grade) (All)

Marketing Strategy A Decision Focused Approach 8th Edition By John Mullins, Orville Walker (Test Bank, 100% Verified Original, A+ Grade)
Marketing Strategy A Decision Focused Approach, 8e John Mullins, Orville Walker (Test Bank, 100% Verified Original, A+ Grade) Marketing Strategy A Decision Focused Approach, 8e John Mullins, Orville...
By eBookSmTb , Uploaded: Oct 04, 2023
$25
Business Management> TEST BANK > Marketing Management A Strategic Decision Making Approach 8th Edition By John Mullins, Orville Walker (Test Bank, 100% Verified original, A+ Grade) (All)

Marketing Management A Strategic Decision Making Approach 8th Edition By John Mullins, Orville Walker (Test Bank, 100% Verified original, A+ Grade)
Marketing Management A Strategic Decision Making Approach, 8e John Mullins, Orville Walker (Test Bank, 100% Verified original, A+ Grade) Marketing Management A Strategic Decision Making Approach, 8e...
By eBookSmTb , Uploaded: Oct 02, 2023
$25
Finance> TEST BANK > Principles of Managerial Finance 11th Edition By Gitman Lawrence (Test Bank, 100% Verified Original, A+ Grade) (All)

Principles of Managerial Finance 11th Edition By Gitman Lawrence (Test Bank, 100% Verified Original, A+ Grade)
Principles of Managerial Finance 11e Gitman Lawrence (Test Bank, 100% Verified Original, A+ Grade) Principles of Managerial Finance 11e Gitman Lawrence (Test Bank, 100% Verified Original, A+ Grade)...
By eBookSmTb , Uploaded: Oct 04, 2023
$20
Finance> TEST BANK > Principles of Managerial Finance 8th Edition (Brief Edition) By Chad Zutter, Scott Smart (Test Bank, 100% Verified Original, A+ Grade) (All)

Principles of Managerial Finance 8th Edition (Brief Edition) By Chad Zutter, Scott Smart (Test Bank, 100% Verified Original, A+ Grade)
Principles of Managerial Finance, Brief Edition, 8e Chad Zutter, Scott Smart (Test Bank, 100% Verified Original, A+ Grade) Principles of Managerial Finance, Brief Edition, 8e Chad Zutter, Scott Smart...
By eBookSmTb , Uploaded: Oct 04, 2023
$20
Statistics> TEST BANK > Statistical Reasoning for Everyday Life 5th Edition By Jeff Bennett, William Briggs, Mario Triola (Test Bank, 100% Verified Original, A+ Grade) (All)

Statistical Reasoning for Everyday Life 5th Edition By Jeff Bennett, William Briggs, Mario Triola (Test Bank, 100% Verified Original, A+ Grade)
Statistical Reasoning for Everyday Life, 5e Jeff Bennett, William Briggs, Mario Triola (Test Bank, 100% Verified Original, A+ Grade) Statistical Reasoning for Everyday Life, 5e Jeff Bennett, William...
By eBookSmTb , Uploaded: Oct 04, 2023
$20
Document information
Connected school, study & course
About the document
Uploaded On
Apr 07, 2021
Number of pages
118
Written in
Additional information
This document has been written for:
Uploaded
Apr 07, 2021
Downloads
0
Views
110






