Chemistry > ASSIGNMENT > CHEM120 Week 5 Concepts: Redox and Organic Chemistry – Download For Revision And Score An A+ (All)
CHEM120 Week 5 Concepts: Redox and Organic Chemistry – Download For Revision And Score An A+
Document Content and Description Below
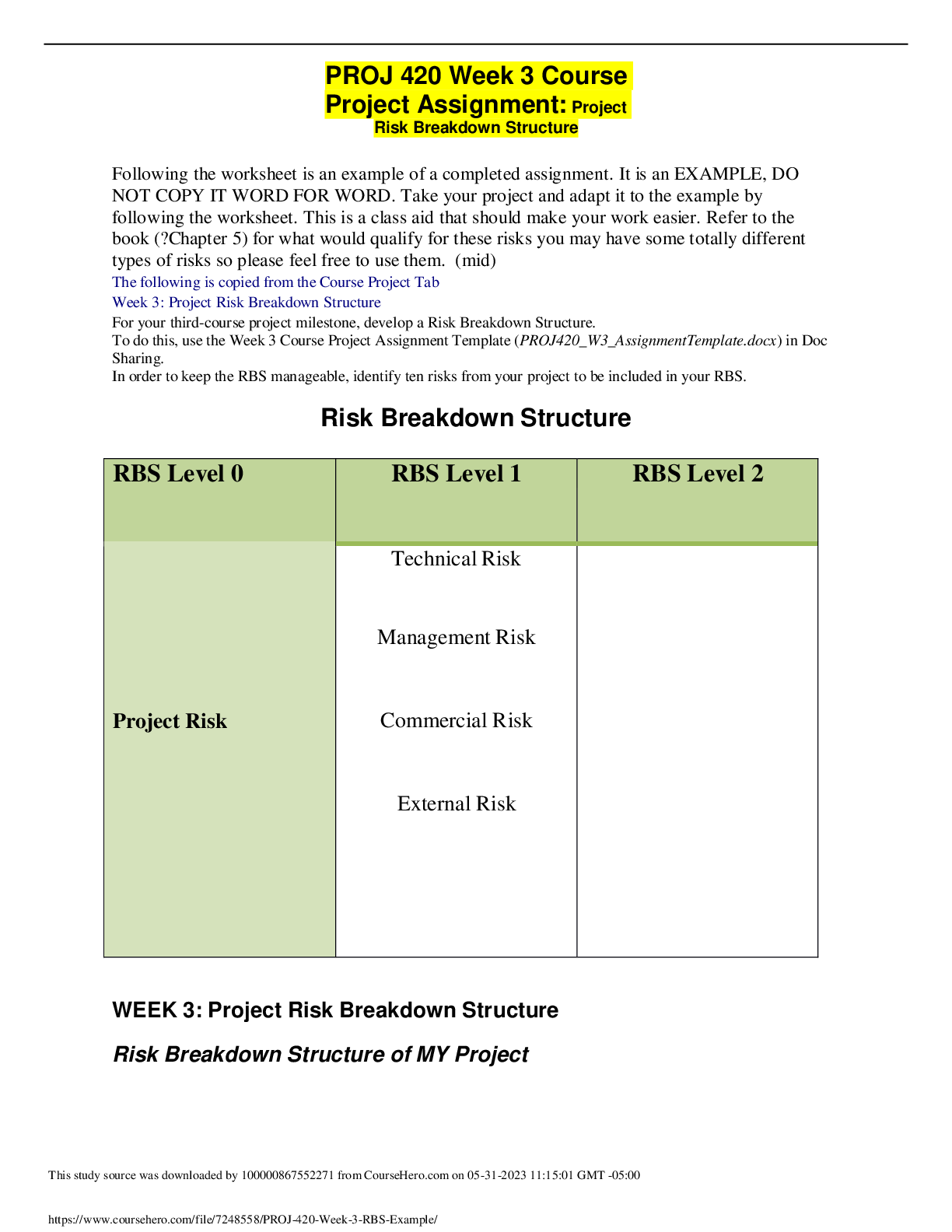
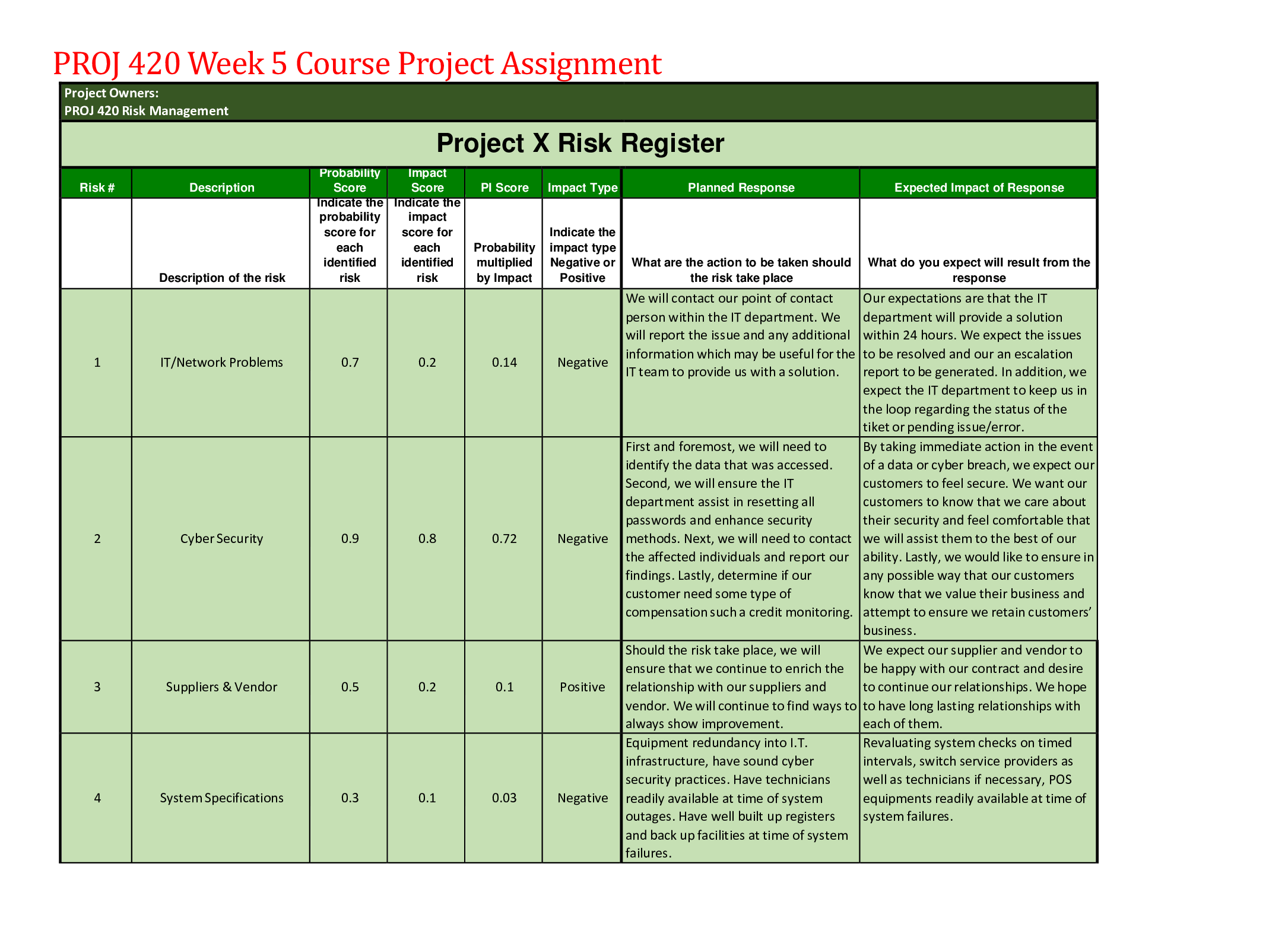
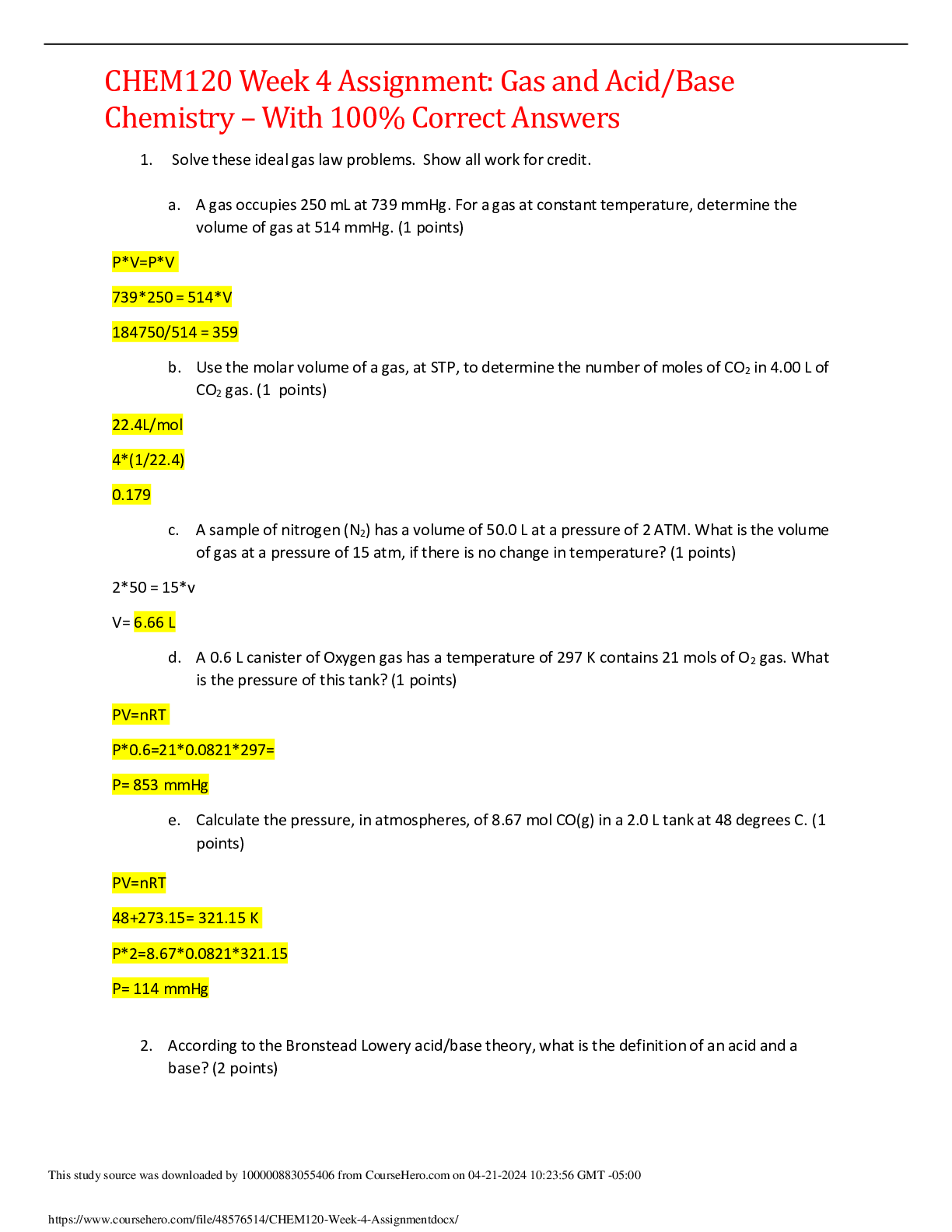
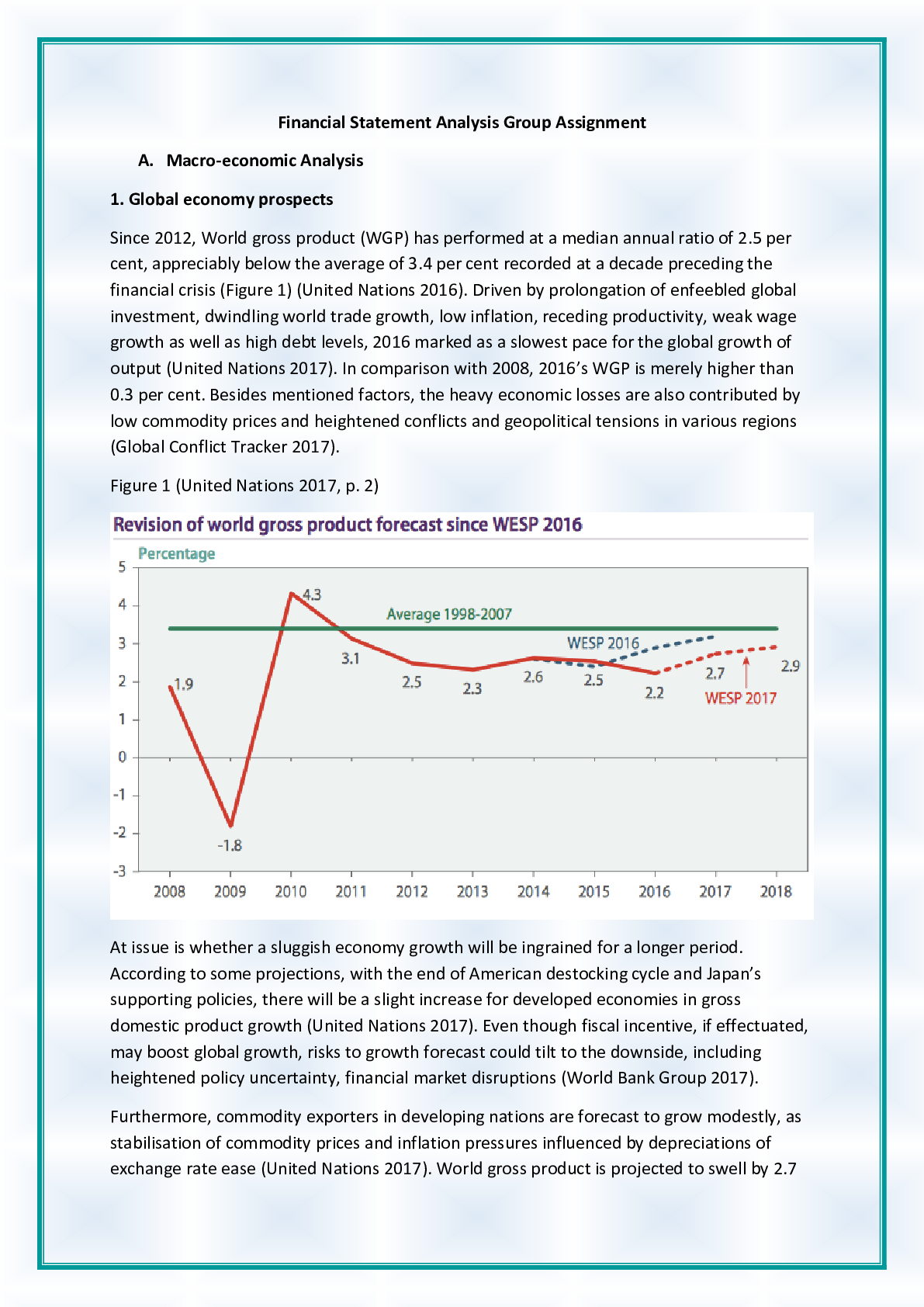
CHEM120 Week 5 Concepts: Redox and Organic Chemistry – Download For Revision And Score An A+ OXIDATION-REDUCTION Reduction and oxidation are intertwined concepts that are important to understand... ing some of the most important chemical reactions in the human body. In this lesson, we will explore the relationship between these terms and the how to identify what chemicals in a reduction/oxidation reaction (redox reaction) are playing which parts. 1. Redox reactions involve a transfer of: ▪ Nucleons ▪ Neutrons ▪ Electrons ▪ Protons 2. If iron reacts with oxygen gas in the reaction 2 Fe(s) + O2(g) →2Fe O(s) , what is oxidized in this reaction? ▪ No oxidation is occurring here ▪ FeO ▪ O2 ▪ Fe REDUCTION AND OXIDATION Reduction and oxidation involves the transfer of electrons between two species within a chemical reaction. Not all chemical reactions involve reduction and oxidation; however, the reactions that involve reduction and oxidation are called Redox reactions. As usual, we need to understand a bit of terminology in order to understand these reactions further. • Oxidation: loss of electrons • Reduction: gain of electrons You may find the word “reduction” a bit of an odd choice for a reaction that involves gaining of electrons. The key is to remember that electrons have a -1 charge, and thus, the more electrons an atom gains, the more negative the charge. To help remember these terms, the mnemonic below can help: LEO the lion goes GER: Loss of Electrons is Oxidation and Gain of Electrons is Reduction In a chemical reaction, oxidation cannot happen without reduction and reduction cannot happen without oxidation. 3. Oxidation involves the loss of electrons while reduction involves the gain of electrons. Consider a reaction where a Li+ is converted into Li. Do you think that this Lithium atom gained or lost an electron? ▪ gained electron ▪ lost electron IDENTIFYING OXIDATION AND REDUCTION We know that electrons have a -1 charge, and we understand that +1 -1 = 0. So, if Li+ gains an electron, this is what cancels out the positive (+1) charge and makes this atom neutral. The rule for oxidation and reduction, based on the gain or loss of electrons is that: • As the charge of a species increases in the positive direction, the species is oxidized. • As the charge of a species decreases, becoming more negative, the species is reduced. A visualization of this relationship appears in the image. Note the direction of the arrows. When the Li+ became Li, we see that we moved from +1 to 0, or downwards, telling us that this process was reduction. As we see, if charges are present, determining reduction and oxidation is as easy as looking at how the charges change. 4. In the following reactions, indicate if the species is oxidized or reduced: ▪ Mg2+ becomes Mg Reduced • becomes O2 Reduced ▪ Fe2+ becomes Fe3+ Oxidized ▪ F becomes F- Reduced ▪ Cu becomes Cu+ Oxidized ▪ H becomes H+ Oxidized OXIDATION AND REDUCING AGENTS Reduction cannot happen without oxidation and oxidation cannot happen without reduction. For example, when a species is oxidized, losing one or more electrons, those electrons must go to another species in the reaction, causing the other species to be reduced. • Oxidizing agents are reduced in a chemical reaction (because oxidizing agents accept electrons) • Reducing agents are oxidized in a chemical reaction (because reducing agents donate electrons) As seen in the image, species A transfers electrons to species B, and thus species A is the reducing agent. You also see that since species A has lost electrons, species A is oxidized. In other words, the species being oxidized is the reducing agent while the species being reduced is the oxidizing agent (species B in this case). In chemical reactions, a common oxidizing agent is oxygen gas, O2. For example, rust forms when O2 reacts with iron, Fe, causing oxidation. 2 Fe(s) + O2(g) → FeO(S) In this reaction, we would consider the O2 to be the oxidizing agent and Fe to be the reducing agent, as electrons were transferred from Fe to O. A common reducing agent is hydrogen gas, H2. In the reaction below, we see how hydrogen gas can reduce the iron, forming Fe from FeO. FeO(S) + H2(g) → Fe +H2O(g) In this reaction, we would consider H2 to be the reducing agent and FeO to be the oxidizing agent as electrons were transferred from the H2 to the Fe in the FeO. Putting together what you have learned in this lesson, you can identify oxidizing and reducing agents. In this way, if you can determine whether a species is gaining or losing electrons, you can determine if the species is oxidized or reduced and thus the reducing agent or oxidizing agent. Consider the reaction between solid zinc and a solution containing copper ions: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) As you can see, the solid Zn donated two electrons to the Cu ion. We know this because you see that Zn went from having no charge (0) to having a positive charge (+2). This tells us that the Zn has lost these electrons. Where did these electrons go? To the Cu ion as the Cu went from a charge of +2 to a charge of 0, indicating a gain of electrons (remember, electrons have a -1 charge, so the more electrons an atom gets, the more negative it becomes). In the image you see that the solid Zn must be the reducing agent as this is the species causing another species, the Cu2+, to become reduced. Once we know the reducing agent, we know that the oxidizing agent must be the other reactant, as reduction cannot happen without oxidation. 5. Choose the correct agent in each of these reactions: ▪ Mg(s) + O2(g) → MgO Select the oxidizing agent: O2(g) ▪ CuO(s) + H2(g) → Cu(s) + H2O(g) Select the reducing agent: CuO(s)H2(g)Cu(s)H2O(g) ▪ 2 Na(s) + Cl2(g) → NaCl(s) Select the reducing agent: 2 Na(s) REDOX ALL AROUND YOU Redox reactions are all around you and play important roles in the environment as well as within our own bodies. One important redox reaction that we all depend on is photosynthesis: 6 CO2 + 12 H2O + light → C6H12O6 + 6 H2O + 6 O2 This is the reaction that produces the oxygen gas we breathe as well as glucose (C6H12O6), the simple sugar that fuels life on this planet. The carbon in CO2 is reduced and the oxygen in water is oxidized. We also see the reverse of this reaction in cellular respiration: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy(ATP) In cellular respiration, the carbon in glucose is oxidized to form CO2 and the oxygen is reduced to form H2O. Cellular respiration is the reaction organisms of all types use to break down glucose for energy in the form of ATP, the chemical energy used by cells. Another important part of cellular energy is nicotinamide adenine dinucleotide, abbreviated as NAD, found in all cells. This important molecule is key to metabolism and carries electrons around the cell to facilitate metabolism. NAD+ + 2H → NADH + H+ As we can see, NAD+ is reduced to form NADH. 6. If a species accepts electrons from another reactant in a chemical reaction, this species is: (select all that apply) ▪ Being reduced ▪ Being oxidized ▪ Reducing agent ▪ Oxidizing agent 7. Which metal is being oxidized based on the reaction? Cu(s) + Ag+(aq) → Cu+(aq) + Ag(s) ▪ Cu(s) ▪ Ag+(aq) ▪ Cu+(aq) ▪ Ag(s) 8. What would be the oxidizing agent in the reaction 2 Ag(s) + O2(g) → Ag2O(s) ▪ Ag(s) ▪ O2(g) ▪ Ag2O(s) ▪ This is not a redox reaction 9. An important reaction in metabolism involves NAD+. In the reaction, what compound would be considered the oxidizing agent: NAD+ + 2 H → NADH + H+ ▪ NAD+ ▪ H ▪ NADH ▪ H+ ORGANIC CHEMISTRY-HYDROCARBONS In this section we turn our attention to the important chemicals found in living things: organic compounds. We will • Examine the importance of carbon in organic chemistry by emphasizing its unique electron arrangement. • Differentiate between structural formulas and naming of saturated and unsaturated hydrocarbons. • Identify and draw condensed and extended structural formulas of simple, cyclic and aromatic hydrocarbons. CARBON BONDING Organic chemistry starts with carbon and the fact that carbons have 4 unpaired valance electrons, based on its position in Group 4 of the periodic table. This allows each C to make 4 bonds. Carbon has the unique ability to bond with itself, unlike other Group 4 elements, making it possible to form chains of carbon. This essential property allows carbon chains to form the basic building blocks of cells. The golden rule of organic chemistry is that C is expected to make 4 bonds. When drawn, each bond is represented by a line between two atoms. Carbon is able to form single, double, or triple bonds due to its four unpaired valence electrons. ▪ Single bonds are represented by one line between two atoms and are one bond ▪ Double bonds are represented by two lines between two atoms ▪ Triple bonds are represented by three lines between two atoms In each instance, each line is a bond and is bond is a line. For example, let us look at the compound Propane. We can see here that each of these carbons is making 4 bonds, all the bonds are single bonds because one line is shown. We can see carbon making single bonds with hydrogen as well as carbon. In total, four bonds are observed on each carbon atom. Propane How about the compound Propene? In the image of Propene, we see a carbon-carbon double bond represented by two lines between carbon atoms. Each line represents one bond, so a double bond counts as two bonds. We see additional single bonds to hydrogen, giving four bonds total on each carbon atom. Propene As shown in the image of Propene, as long as exactly four total bonds are present on carbon, it can be any combination of single, double, or triple bonds. In the example above, there is one double bond and two single bonds on each of the carbons, giving four bonds total. This can also be achieved with four single bonds, or a triple bond and a single bond, as shown in the image of Butyne. 10. Two carbon atoms are bonded directly together, and each have one single bond to a hydrogen. This will require three more bond(s) to meet the bonding requirements of carbon and will be accomplished by a triple bond. ▪ Because each carbon has one bond, it will need three other bonds to meet the four bond requirement of carbon. This will be accomplished by a triple bond. HYDROCARBONS Hydrocarbons (compounds made only of carbon and hydrogen) are the backbone of organic chemistry and are key to the naming rules for organic compounds in general. Naming hydrocarbon chains is simple if we follow the naming rules below. The key term here is carbon chain. We determine the length of the carbon chain by counting the number of carbons linked together in a line (if there is a break in the chain, each chain is counted separately). In the image, three carbons are connected together in the carbon chain. This will serve as the root for naming the organic structure. This information should be committed to memory. When all single bonds are present, the hydrocarbon is known as an alkane. The alkanes are named by first identifying the correct root and adding the suffix –ane. The carbon compound in the image would properly be named propane: • Three carbons in the carbon chain gives the prop- root • Only carbon-carbon single bonds in the carbon chain means this is an alkane, and the name will have the -ane ending These types of hydrocarbons are not reactive on their own but can store tremendous energy in their carbon-carbon bonds and are often used as fuel sources. You might burn propane, shown in the image, in your gas grill, or butane on your camp stove. Your car might even have a high-octane engine. This naming convention should be practiced and mastered, as it is the basis of all other organic naming. As additional groups are added to the structure to give different functions, the suffix changes. Number of carbons in the chain Root 1 Meth- 2 Eth- 3 Prop- 4 But- 5 Pent- 6 Hex- 7 Hept- 8 Oct- 9 Non- 10 Dec- 11. How many carbons are in butane, often used in cooking oil? ▪ 1 ▪ 2 ▪ 3 4 12. How many carbons in methane, a common greenhouse gas expelled from cattle? 1 ▪ 2 ▪ 3 ▪ 4 EXTENDED AND CONDENSED STRUCTURES IN ORGANIC CHEMISTRY The structures of organic compounds can be shown in either extended or structural formulas. • Extended structural formulas show every atom and each bond is represented by line(s) • Condensed structural formulas show all atoms, but few or no bonds. So far, we have examined only extended structural formulas; these same structures can also be represented with condensed structural formulas, which are easier to type. The condensed structural formulas show the carbons and all attached atoms, written similarly to the molecular formula. Because this is a structural formula, this is written for each carbon individually to show the bonding and attachments on each carbon In the chain. In many cases, each carbon is directly bonded to the following carbon. We will note two important exceptions to this rule later. Structural depiction, showing how each carbon is bonded, is essential in organic chemistry because even small changes will have large effects on the function of the molecule. Molecules called isomers (“iso” meaning same, and “mer” meaning parts) will have identical molecular formulas but different structures. This gives different boiling points to isopropyl alcohol and propanol, though both have the molecular formula C3H8O and the same molecular weight. For this reason, the structural formulas are used in organic chemistry to show structure and naming, rather than the molecular formulas which do not provide this information. Propanol Isopropanol Extended structural formula Condensed structural formula CH3CH2CH2OH CH3CHOHCH3 Molecular formula C3H8O C3H8O Boiling point 97.2 ºC 82.2 ºC The extended structural formula can be considered to determine the condensed formula. Looking at the image, each carbon and its attachments are written in order. The first carbon has three hydrogens attached, so it will be written as CH3 in the condensed formula. The second carbon, attached to the first, has only two H, so it will be written as CH2. The third also has two H attached and will also be written as CH2. The final carbon has three H and will be written as CH3. Placing these in order from left to right, the condensed formula is CH3CH2CH2CH3, based on the provided extended formula. To decipher condensed structural formulas to determine the extended structural formula uses a little more background knowledge. We consider the formulas of the carbons first, one carbon at a time, and must determine where the bonds are and if the bonds are single, double, or triple. We will have to deduce this based on the bonding requirement for the atoms in the formula. Atom Required number of bonds Carbon (C) 4 Nitrogen (N) 3 Oxygen (O) 2 Hydrogen (H) 1 The carbon is typed first and its attached atoms are typed immediately following. We know in organic chemistry each carbon will have four total bonds and will bond in carbon chains. In the condensed structural formula above, CH3 has three hydrogen atoms attached to one carbon; this means one more bond is needed to fulfil the four-bond requirement of carbon. That bond must be located between this carbon and the next in the chain, in order for the carbon chain to exist. The next carbon in the chain above has a condensed structural formula of “CH2”. This means that it has two hydrogens attached to this carbon; from our previous deduction, we also know this carbon is bound to the first carbon. That gives a total of three bonds, when four are required, so this carbon must be attached to the following carbon. The pattern you will notice is that when there are carbon-carbon single bonds, there are three hydrogens on end carbons and two on middle carbons. For double bonds, there is one less hydrogen on end and middle carbons where the double bond is located: two H on end carbons, and one on middle carbons. In triple bonds, there are two fewer H: one on end carbons and none on middle carbons. Carbon-carbon bond type Carbon location Number of H attached Single End Middle 3 2 Double End Middle 2 1 Triple End Middle 1 0 13. Which of the following will show the difference in structure between isopropanol (rubbing alcohol) and propanol? Select all that apply. ▪ Condensed structural formula ▪ Molecular formula ▪ Molecular weight ▪ Extended structural formula i. The way to determine a difference in structure is through a structural formula such as the extended structural formula, or the condensed structural formula. Molecular formula or molecular mass will be identical for isomers, which have different structures but identical formulas SATURATED AND UNSATURATED HYDROCARBONS The alkanes are the simplest hydrocarbons containing only carbon-carbon single bonds. We learned earlier that carbons can contain double or triple bonds between them as well. This change in structure will require a change in naming. Alkanes are considered saturated hydrocarbons because they contain only single bonds, and thus have the maximum number of hydrogen atoms bound. This is considered fully saturated with hydrogen. The alkenes and alkynes have double and triple bonds, respectively, and thus are unsaturated hydrocarbons. When the double or triple bond is added, there is a subsequent loss of hydrogen in the structure to maintain four total bonds on each carbon atom. There is a lower number of hydrogens in the unsaturated structures. In naming unsaturated hydrocarbons, we will use the same roots for the number of carbons in the chain, and change the suffix to describe the bond: Hydrocarbon type Suffix Bonding type Alkane -ane No C to C double or triple bonds C-C Alkene -ene C to C double bond C=C Alkyne -yne C to C triple bond C≡C 14. Which of the following contains triple bonds? ▪ Butyne ▪ butane, butene, butyne ▪ butene ▪ butane 15. Determine if the hydrocarbon is saturated or unsaturated. ▪ Alkene unsaturated ▪ Alkyne unsaturated ▪ Alkane saturated CYLIC AND AROMATIC COMPOUNDS The final class of hydrocarbons we will consider are the cyclic and aromatic compounds. CYCLIC HYDROCARBONS Cyclic hydrocarbons are saturated hydrocarbons with polygon structures: there are no carbons on the end, rather each carbon is connected to another carbon. These structures are named by the prefix “cyclo-” followed by the root and suffix. For example, cyclohexane is a six-carbon alkane, arranged in a hexagon shape rather than a line. Cyclohexane AROMATIC HYDROCARBONS Aromatic hydrocarbons are polygonal unsaturated hydrocarbons with special arrangements of double bonds that allow unique functions; often these hydrocarbons have a scent, thus the name “aromatic.” Many of these compounds have historical significance and are referred to by common names. ▪ Benzene is a common laboratory solvent and the basis for many other aromatic structures. This cyclic structure has six carbons arranged in a hexagon, and double bonds located between every other carbon. You will notice it looks similar to cyclohexane, which also forms a hexagon. Cyclohexane is saturated, whereas benzene is unsaturated. ▪ Naphthalene is an aromatic compound found in moth balls. This is a bi-cyclic structure containing two carbon rings. Notice the structure looks like two benzene rings joined together. ▪ Phenol, another derivative of benzene, is an aromatic compound found in disinfecting sprays. The structure is benzene with an additional group called a hydroxyl group, -OH, attached to a carbon. 16. Name the extended structural formula: ▪ CYCLOPENTANE ▪ NAPHTHALENE ▪ BENZENE ▪ CYCLOHEXANE 17. Classify these extended structures as Aromatic or Cyclic Hydrocarbons: ▪ CYCLIC HYDROCARBON ▪ AROMATIC HYDROCARBON ▪ AROMATIC HYDROCARBON ▪ CYCLIC HYDROCARBON 18. Which of the following contains double bonds? ▪ CH3CH2CH2CH3 ▪ hexyne ▪ methane ▪ ethene ▪ CH2CHCH2CH2CH3 19. Drag the compound to its correct classification ▪ Aromatic Hydrocarbon: i. Benzene ii. Phenol ▪ Cyclic Hydrocarbons i. Cyclopentane ii. Cycloheptane 20. Which of the following is an unsaturated hydrocarbon containing 7 carbons? ▪ mutane ▪ pentene ▪ hexane ▪ benzene ▪ heptene 21. Determine if the compound is saturated or unsaturated: ▪ Benzene Unsaturated ▪ Propyne Unsaturated ▪ Methane Saturated ▪ Propane Saturated 22. The compound with a condensed structure of CH3CCCH2CH3 has 5 carbons and has a triple bond. The name will be pent yne. 23. Choose the correct condensed structure from the name: hexene. ▪ CHCCH2CH3 ▪ CH3CHCHCH2CH3 ▪ CH3CH2CH2CH2CH2CH3 ▪ CH3CH2CHCHCH2CH3 o Hexene will have six carbons and a double bond, from the “hex” root meaning six and the –ene suffix. The double bond in a condensed structure has one less H on each of the involved carbons. 24. Which is the correct extended structural formula for CH2CH2? 25. Choose the name and condensed formula to match the extended structure. ▪ Name: Butene ▪ Extended structural formula: CH3CHCHCH3 26. Due to its electron configuration and valence shell electrons, carbon can form 4 bonds, nitrogen can form 3 bonds, and oxygen can form 2 bonds. 27. Carbons can form carbon-carbon links, also known as , that can be linear or cyclic. ▪ carbon chains ▪ root number ▪ carbon bridges ▪ carbon connections ▪ triple bonds [Show More]
Last updated: 2 weeks ago
Preview 1 out of 16 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 22, 2024
Number of pages
16
Written in
Additional information
This document has been written for:
Uploaded
Apr 22, 2024
Downloads
0
Views
10