BioChemistry > QUESTIONS & ANSWERS > 150 Biochemistry Exam 1 practice questions with correct answers. Rated A. 2022/2023 updates (All)
150 Biochemistry Exam 1 practice questions with correct answers. Rated A. 2022/2023 updates
Document Content and Description Below
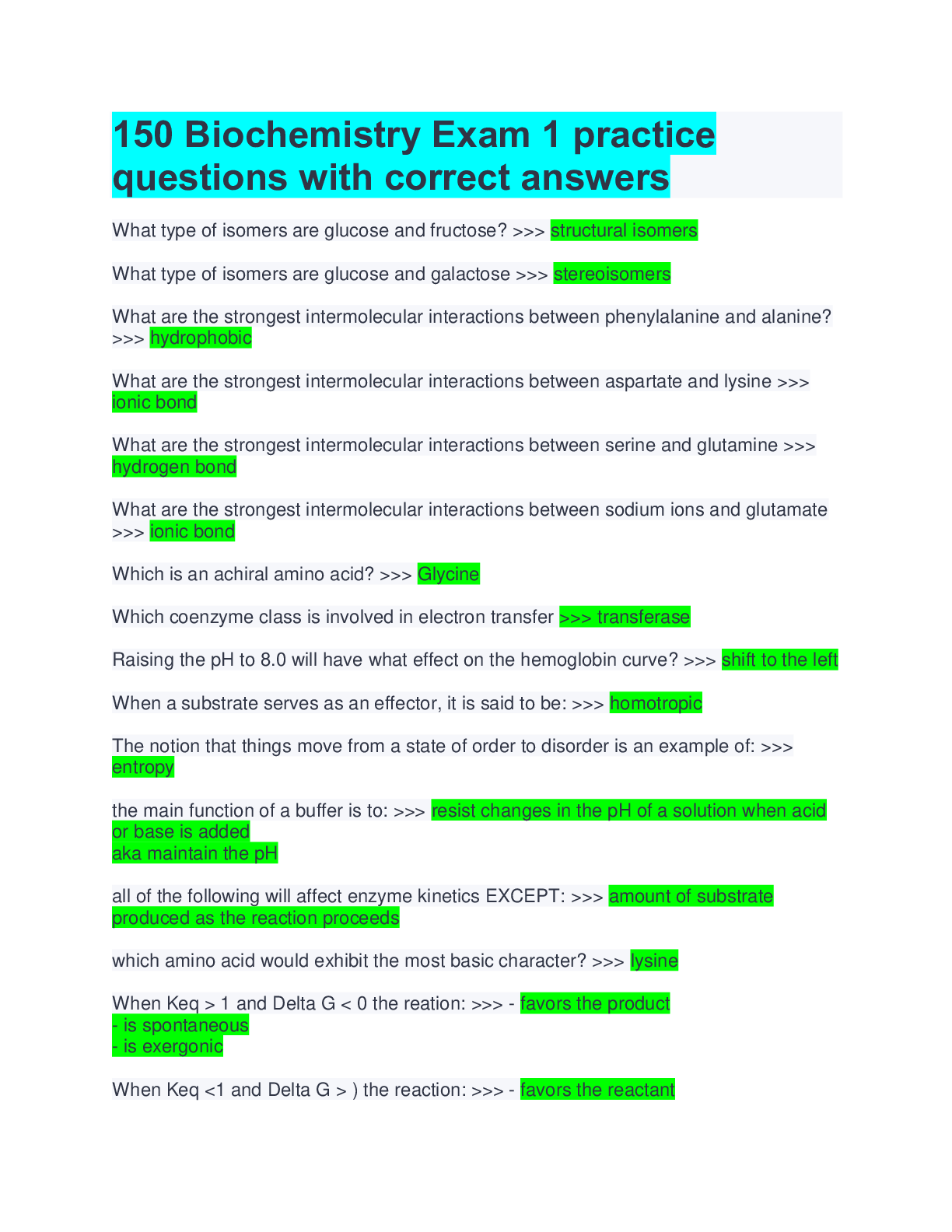
What type of isomers are glucose and fructose? >>> structural isomers What type of isomers are glucose and galactose >>> stereoisomers What are the strongest intermolecular interactions between phen... ylalanine and alanine? >>> hydrophobic What are the strongest intermolecular interactions between aspartate and lysine >>> ionic bond What are the strongest intermolecular interactions between serine and glutamine >>> hydrogen bond What are the strongest intermolecular interactions between sodium ions and glutamate >>> ionic bond Which is an achiral amino acid? >>> Glycine Which coenzyme class is involved in electron transfer >>> transferase Raising the pH to 8.0 will have what effect on the hemoglobin curve? >>> shift to the left When a substrate serves as an effector, it is said to be: >>> homotropic The notion that things move from a state of order to disorder is an example of: >>> entropy the main function of a buffer is to: >>> resist changes in the pH of a solution when acid or base is added aka maintain the pH all of the following will affect enzyme kinetics EXCEPT: >>> amount of substrate produced as the reaction proceeds which amino acid would exhibit the most basic character? >>> lysine When Keq > 1 and Delta G < 0 the reation: >>> - favors the product - is spontaneous - is exergonic When Keq <1 and Delta G > ) the reaction: >>> - favors the reactant- is not spontaneous - is endergonic non-competitive inhibition: >>> decrease the vmax Km stays the same glucose and galactose form: >>> lactose glucose and fructose form: >>> sucrose glucose and glucose form: >>> maltose What is an epimer of glucose? >>> galactose What is the 1st Law of Thermodynamics? >>> Energy cannot be created or destroyed only converted into different forms Gibbs Free Energy Formula: >>> DeltaG = DeltaH - TDeltaS True or false: spontaneous and instantaneous are the same thing >>> false What is Hess's Law >>> as long as the reaction ends up in a egative number the process will occur spontaneously Endogonic reactions are ______________ and Exergonic reactions are __________ >>> anabolic; catabolic When the free energy of a reaction is zero: >>> equilibrium What do enzymes do to the energy required for activation? >>> lowers energy Is the enzyme consumed during a reaction? >>> No Acids with a high Ka are >>> strong (wants to lose hydrogen) Acids with a low Ka are >>> weak (wants to gain a hydrogen) High Ka = what pKa? >>> Low pKa (strong acid) Low Ka = what pKa? >>> High oKa (weak acid) What is the range that buffers work well in? >>> plus or minus 1 of pka of pH What is a good buffer range for pKa = 10? >>> pH range 9-11 What is it called if the pH is < pKa >>> protinatedWhat is it called if the pH is > pKa >>> deprotinated competitve inhibition: >>> increase Km Vmax stays the same uncompetitive inhibition >>> change in Vmax and Km (parralel lines on graph) ATP synthase belongs to which class of enzymes? >>> Translocase Enantiomers are: >>> Mirror images of one another but cannot superimpose on one another Alcohol dehydrogenase requires NAD+ for catalytic activity. NAD+ functions as a: >>> coenzyme-cosubstrate Which pKa exhibits the most basic character? >>> a higher one Enzymes can be regulated by all of the following EXCEPT: >>> Degradation What is a structural isomer of glucose? >>> Fructose Lactose belongs to which class of enzymes >>> hydrolase Which enzyme uses wate to remove a phosphate? >>> phosphatase Maximum buffering capacity occurs when? >>> pKa = pH in a double-stranded DNA, adenine hydrogen bonds to: >>> Thymine stereoisomers are: >>> same molecular formula but differ in how they are arranged type of functional group that forma the bond between amino acids in a peptide: >>> amide What functional group does NOT contain a carbonyl? >>> ether all proteins contain: >>> carbon, hydrogen, oxygen, nitrogen The Michaelis-Menten equation: >>> V0 = Vmax [S]/Km + [S] Lipase belongs to which class of enzyme? >>> Hydrolase An example of a rich electron carrier for enzymatic reactions is: >>> NADHHow many subunits make up the quaternary structure of a protein/ >>> 2 or more Which of the following would make up a strand of DNA? >>> ATCG The lines of a Lineweaver-Burk plot displaying competitive inhibition will: >>> decrease affinity of the enzyme for the substrate When [S] is much greater than Km the reaction rate is: >>> Zero order alpha and beta forms of the disaccharide lactose are called: >>> anomers Tyromsine, phenylalanine, and tryptophan all belong to which class of amino acids? >>> Aromatic A coenzyme that is found in oxidoreductase reactions is >>> NADH Enzyme that requires ATP? >>> Ligase An example of a nonpolar branched-chain amino acid is: >>> Isoleucine All proteins contain what bonds? >>> Hydrogen bonds The Hb oxygen binding curve is: >>> sigmoidal due to cooperativity When [S] = Km then ______ >>> the velocity is first order Which of the following statements is NOT true regarding O2 binding affinity? >>> Mb is influenced by pO2 An enzyme that needs a nonprotein cofactor is termed: >>> holoenzyme hexokinase and glucokinase belong to which class of enzymes? >>> Transferase Which of the following is not a protein? >>> cellulose Amino acid that is considered the "helix breaker" >>> glycine glucose and galactose are: >>> epimers what is the strongest intermolecular force between glutamate and lysine in a protein? >>> ionic bonding Higher concentration of CO2 will ahve what affect? >>> Hb has a lower affinity for oxygen If Keq=1 then ____ >>> Delta G = 0Which amino acid contains an amide for its R-group? >>> asparagine Which has the most acidic pKa >>> 2.3 Enzymes are greatly affected by all of the following EXCEPT: >>> Gibbs free energy Enzyme class that cleaves peptide and glycosidic bonds using water: >>> hydrolase In an oxidoreductase reaction, NAD+ would serve as a: >>> coenzyme oxidizing agent Amino Acid attached to the heme ring of Hb >>> Histidine Which amino acid has neither L or D form? >>> Glyci [Show More]
Last updated: 1 year ago
Preview 1 out of 9 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 18, 2022
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Sep 18, 2022
Downloads
0
Views
131