Biology > QUESTIONS & ANSWERS > Arizona State University BIO 101 Bio Exam One Study Guide Bio 11 Exam 1 Practice (All)
Arizona State University BIO 101 Bio Exam One Study Guide Bio 11 Exam 1 Practice
Document Content and Description Below
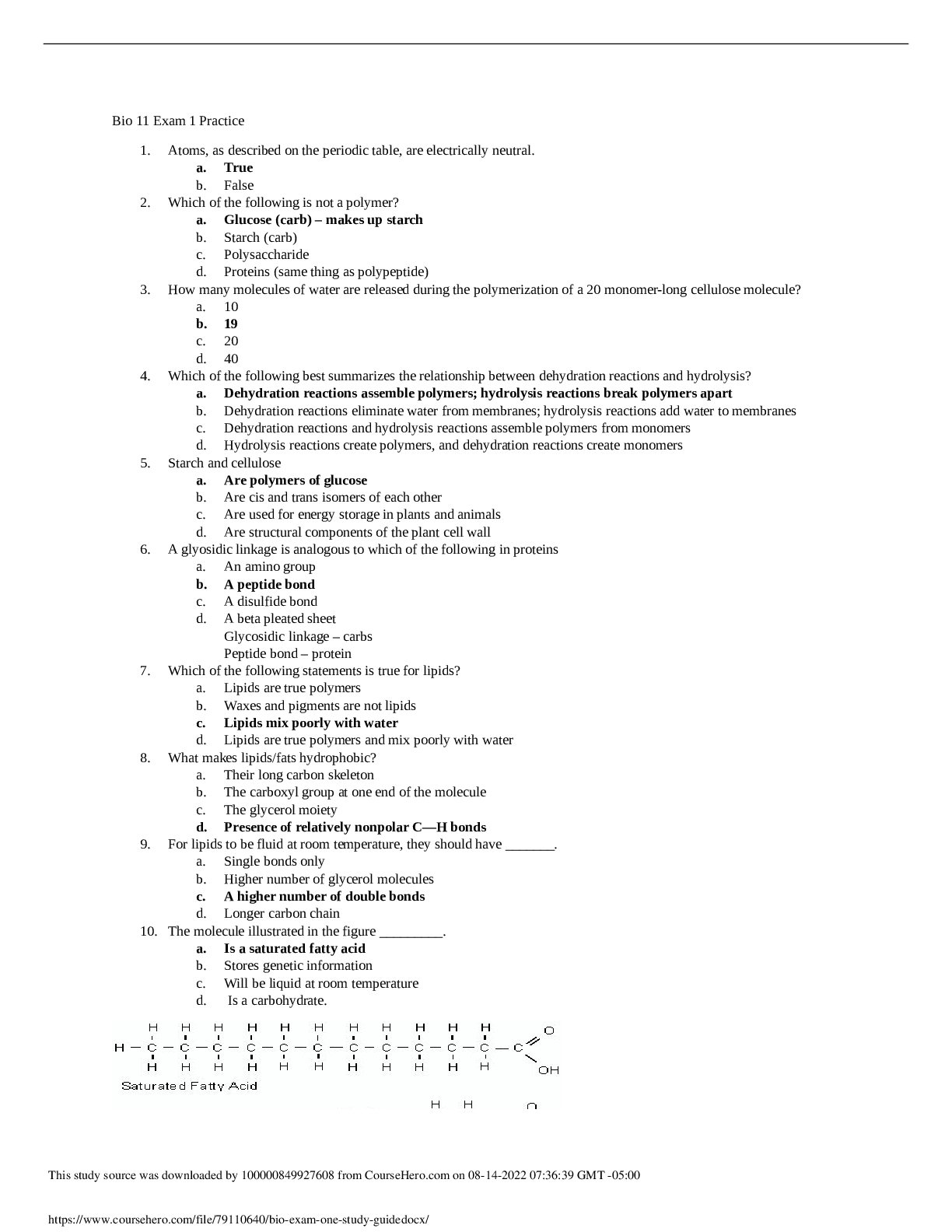
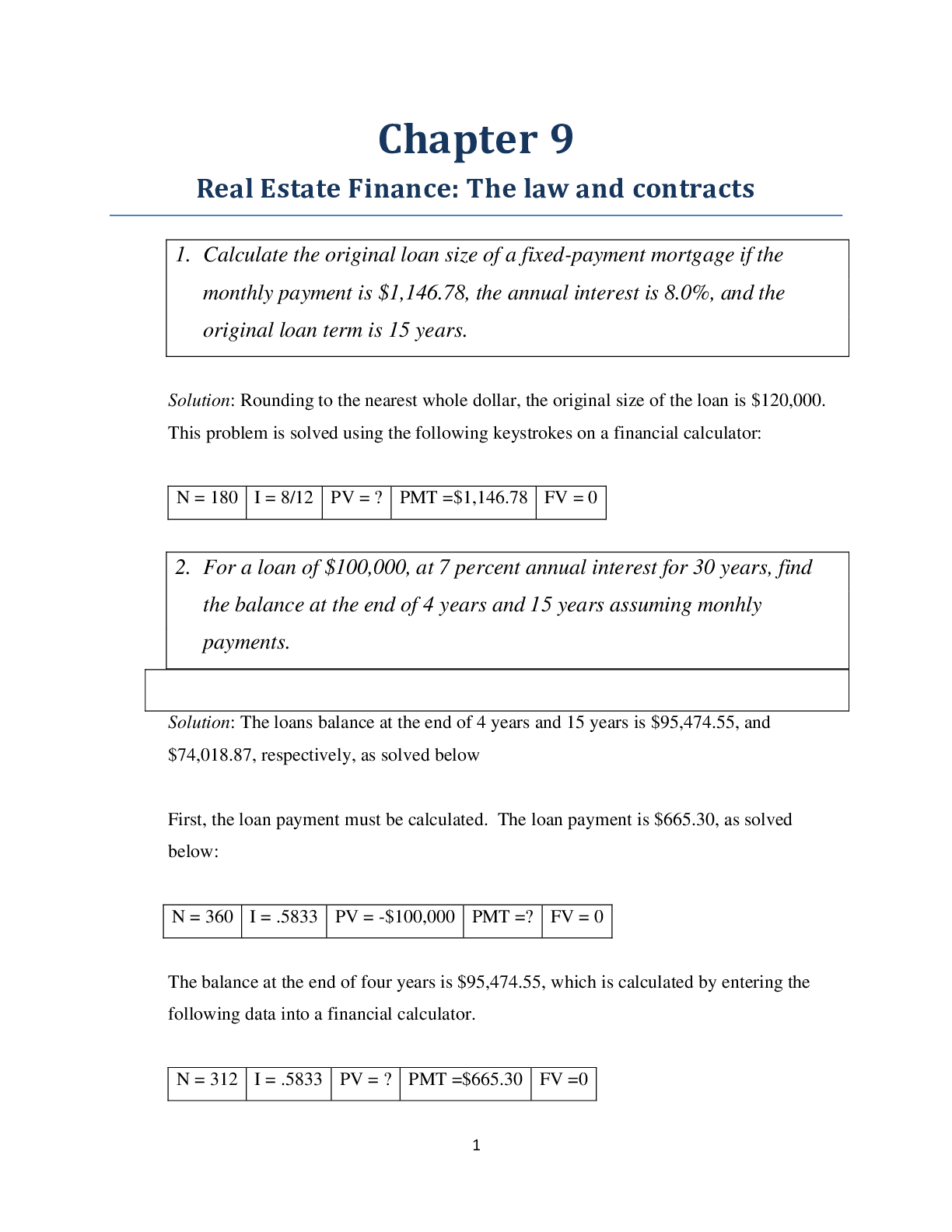
Arizona State University BIO 101 Bio Exam One Study Guide Bio 11 Exam 1 Practice 1. Atoms, as described on the periodic table, are electrically neutral. a. True b. False 2. Which of the followi... ng is not a polymer? a. Glucose (carb) – makes up starch b. Starch (carb) c. Polysaccharide d. Proteins (same thing as polypeptide) 3. How many molecules of water are released during the polymerization of a 20 monomer-long cellulose molecule? a. 10 b. 19 c. 20 d. 40 4. Which of the following best summarizes the relationship between dehydration reactions and hydrolysis? a. Dehydration reactions assemble polymers; hydrolysis reactions break polymers apart b. Dehydration reactions eliminate water from membranes; hydrolysis reactions add water to membranes c. Dehydration reactions and hydrolysis reactions assemble polymers from monomers d. Hydrolysis reactions create polymers, and dehydration reactions create monomers 5. Starch and cellulose a. Are polymers of glucose b. Are cis and trans isomers of each other c. Are used for energy storage in plants and animals d. Are structural components of the plant cell wall 6. A glyosidic linkage is analogous to which of the following in proteins a. An amino group b. A peptide bond c. A disulfide bond d. A beta pleated sheet Glycosidic linkage – carbs Peptide bond – protein 7. Which of the following statements is true for lipids? a. Lipids are true polymers b. Waxes and pigments are not lipids c. Lipids mix poorly with water d. Lipids are true polymers and mix poorly with water 8. What makes lipids/fats hydrophobic? a. Their long carbon skeleton b. The carboxyl group at one end of the molecule c. The glycerol moiety d. Presence of relatively nonpolar C—H bonds 9. For lipids to be fluid at room temperature, they should have _______. a. Single bonds only b. Higher number of glycerol molecules c. A higher number of double bonds d. Longer carbon chain 10. The molecule illustrated in the figure _________. a. Is a saturated fatty acid b. Stores genetic information c. Will be liquid at room temperature d. Is a carbohydrate. 11. The molecule shown in the figure is a __________. a. Fatty acid b. Steroid – 4 rings c. Triacylglycerol d. Phospholipid 12. You disrupt all hydrogen bonds which disrupts the AA interactions in a protein. What level of structure will be preserved? a. Primary (PRIMARY + HYDROGEN = SECONDARY) b. Secondary c. Tertiary d. Quaternary 13. The chemical reaction illustrated _________. a. Is a hydrolysis reaction b. Results in a peptide bond c. Joins two fatty acids together d. Links two polymers to form a monomer 14. The relation between amino acid and polypeptide is similar to the relation between ____. – amino acids make up polyp. And mono. Make up poly. a. Monosaccharide and polysaccharide b. Triglycerides and sterols c. Phospholipid and plasma membrane d. Glycogen and glucose 15. Which of the following includes all others in the list? a. Disaccharide b. Polysaccharide c. Starch d. Carbohydrate 16. The kind and number of bonds an atom can form depends on _______. a. Its atomic number b. Its electronic configuration c. Its atomic mass d. The number of particles in its nucleus 17. A carbon atom has six electrons however, its valence is four. This is because the carbon atom _______. a. Donates its 2 electrons to another atom b. Shares its 2 electrons and bonds with another atom c. Has 4 electrons in its first shell and 2 in its second shell d. Has only 2 electrons in its first shell and 4 in the second 18. Why are hydrocarbons insoluble in water? a. The majority of their bonds are polar covalent carbon to hydrogen linkages b. The majority of their bonds are nonpolar covalent carbon to hydrogen linkages c. They exhibit considerable molecular complexity and diversity d. They are less dense than water 19. Which of the following statement describes geometric isomers? a. Two molecules in which molecules are connected on the same order, but differ by rotational position b. They are mirror images of each other c. They are molecules that share the same atomic formula but differ in their structure d. They have the same chemical properties 20. The kind and number of bonds an atom can form depends on _______. a. Its atomic number b. Its electron configuration c. Its atomic mass d. The number of particles in its nucleus 21. A carbon atom is most likely going to form what kind of bond(s) with other atoms? a. Ionic b. Hydrogen c. Covalent d. Ionic bonds, covalent bonds, and hydrogen bonds 22. The following figure shows glucose and fructose. Why types of isomers are they? a. Isotopes b. Enantiomers c. Geometric isomers d. Structural isomers 23. In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by __________. a. Hydrogen bonds b. Nonpolar covalent bonds c. Polar covalent bonds d. Ionic bonds 24. The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called? a. A covalent bond b. A hydrogen bond c. An ionic bond d. A van der Waals interaction 25. The partial negative charge in a molecule of water occurs because a. The oxygen atom donates an electron to each of the hydrogen atoms b. The electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus c. The oxygen atom has two pairs of electrons in its valence shell that are not neutralized by hydrogen atoms d. One of the hydrogen atoms donates an electron wo the oxygen atom 26. The loss of water from a plant by transpiration cools the leaf. Movement of water in transpiration requires both adhesion to the conduction walls and wood fibers of the plany and cohesion of the molecules to each other. A scientist wanted to increase the rate of transpiration of a crop species to extend its range into warmer climates. The scientist substituted a nonpolar solution with an atomic mass similar to that of water for hydrating the plants. What do you expect the scientist’s data will indicate from this experiment? a. The rate of transpiration will be the same for both water and the nonpolar substance b. The rate of transpiration will be slightly lower with the nonpolar substance as the plant will not have evolved with the nonpolar compound c. Transpiration rates will fall to zero as nonpolar compounds do not have the necessary properties for adhesion and cohesion d. Transpiration rates will increase as nonpolar compounds undergo adhesion and cohesion with wood fibers more readily than water 27. Water is considered a polar molecule because a. The molecule is held together by hydrogen bonds b. The tendency of hydrogen to share elections unevenly forms two “poles” of charge in the molecule c. The tendency of oxygen to share electrons unevenly forms two “poles” pf charge in the molecule d. Two of the above e. All of the above 28. Ionic bonds a. Are rooted in the attraction between 2+ polar atoms b. Form tightly-packed structures because of the close sharing of electrons c. Occur between two or more electronegative atoms d. Are rooted in unequal sharing of electrons, this charging the atoms into ion s e. Are rooted in electrical (charge) attraction between tow atoms that have gained or lost electrons 29. Which of the following items is the strongest base? a. pH 13 b. pH 6.7 c. pH 4.0 d. pH 2 30. Which item has fewer hydrogen ions? a. pH 13 b. pH 6.7 c. pH 4.0 d. pH 2 31. Which of the following functional groups are present in the molecules? Circle all that apply. a. Carbahol b. Amino c. Hydroxide d. Carbonyl e. Carboxyl 32. How many pairs of electrons does carbon need in order to complete its valence shell? a. 1 b. 2 c. 3 d. 4 e. 5 33. What is pH a measure of? 34. Why makes carbon such a foundational element? 35. There are 4 major macromolecules in the body. List all four of them. Then, pick 2 to describe their structures and functions. 36. Compare and contrast starch and glycogen. 37. What 2 ions does pure water disassociate into? 38. Using the characteristics of life explain why a tractor is not alive 39. What is the difference between hydrogen bonding and polar covalent bonds in terms of water molecules? 40. Explain what the difference between an acid or base is. 41. Label as many functional groups on the molecule as possible. [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 14, 2022
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Aug 14, 2022
Downloads
0
Views
28










 Correct Study Guide, Download to Score A.png)



