Pharmacology > STUDY GUIDE > NR565 Week 1 Study Outline( Complete Solution Rated A)Top score (All)
NR565 Week 1 Study Outline( Complete Solution Rated A)Top score
Document Content and Description Below
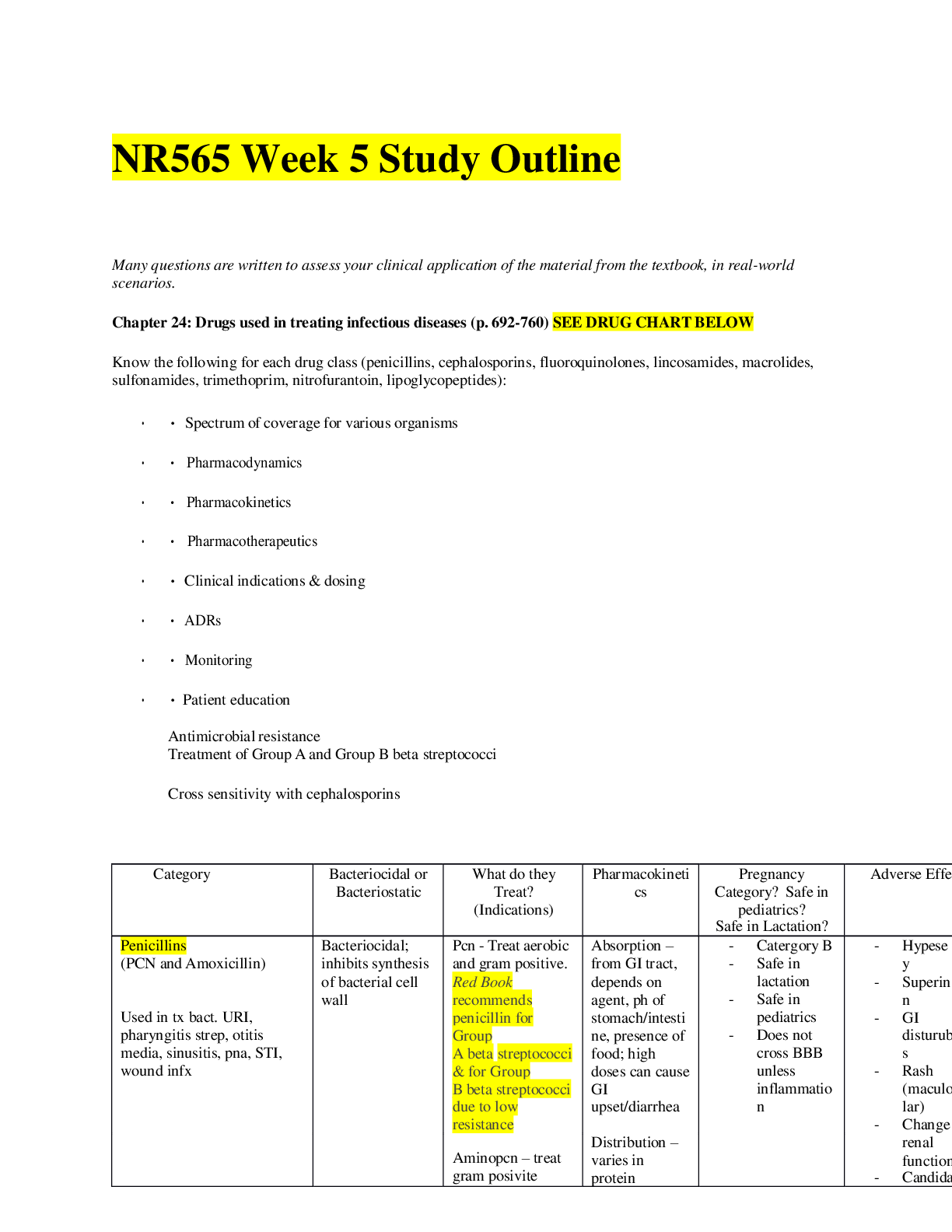
NR565 Week 1 Study Outline Chapter 2: Review of Basic Principles of Pharmacology How Drugs are Developed • Drugs are developed by pharmaceutical companies to help patients and to make money • The ... early part of the drug development process is called the preclinical stage • Pharmaceutical companies will identify a drug target, starting sometimes with ingredients isolated from a plant (or organism in the case of antibiotics) with desirable medicinal properties, sometimes with a molecular target identified in the body to produce the desired response, and sometimes with a disease in need of treatment. • Many drugs are examined as pharmaceutical companies seek the elusive perfect drug with just the right combination of properties. Preclinical studies are performed on cells, isolated tissues and organs, and in laboratory animals to identify promising compounds • Drugs approved by the Food and Drug Administration (FDA) must be both safe and effective and are screened by pharmacologists specializing in various aspects of drug activity. • Ideally, drugs will produce their desired effects at dosages well below those needed to produce toxicity. • During the clinical stage of new drug development, pharmaceutical companies must establish the safety and effectiveness of new products in humans. • Phase I clinical trials typically establish biological effects as well as safe dosages and pharmacokinetics in a small number of healthy patients. o During phase II clinical trials, new drugs are used to treat disease in a small number of patients and to establish the n potential of the drug to improve patient outcomes. • If the drug still looks promising, phase III clinical trials will compare the new medication to standard therapy in a larger number of patients studied by at sites across the country. • New drugs must be at least as good as, and it is hoped better than, other available therapies. Throughout the process, pharmaceutical companies work with the FDA. • After being approved by the FDA, drugs are continuously monitored through post-marketing surveillance, in which health professionals are encouraged to report adverse events, which are studied by both pharmaceutical companies and the FDA [Show More]
Last updated: 1 year ago
Preview 1 out of 26 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 15, 2022
Number of pages
26
Written in
Additional information
This document has been written for:
Uploaded
Mar 15, 2022
Downloads
0
Views
45











 (1).png)