BioChemistry > EXAM > BIS 102 Biochemistry. University of California. Midterm1 Version A_KEY. With working and solutions (All)
BIS 102 Biochemistry. University of California. Midterm1 Version A_KEY. With working and solutions
Document Content and Description Below
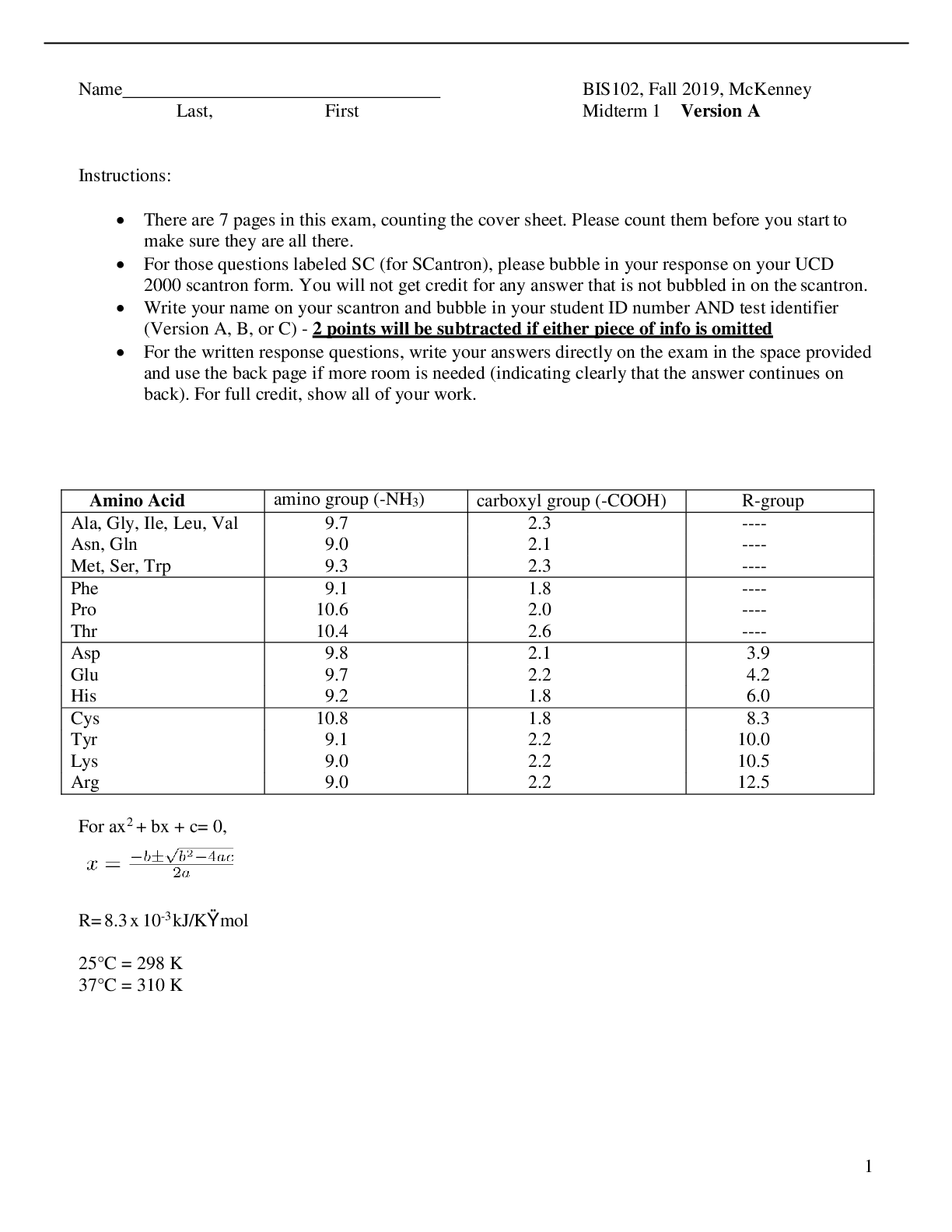
Name BIS102, Fall Last, First Midterm 1 Version A Instructions: • There are 7 pages in this exam, counting the cover sheet. Please count them before you start to make sure they are all t... here. • For those questions labeled SC (for SCantron), please bubble in your response on your UCD 2000 scantron form. You will not get credit for any answer that is not bubbled in on the scantron. • Write your name on your scantron and bubble in your student ID number AND test identifier (Version A, B, or C) - 2 points will be subtracted if either piece of info is omitted • For the written response questions, write your answers directly on the exam in the space provided and use the back page if more room is needed (indicating clearly that the answer continues on back). For full credit, show all of your work. Amino Acid amino group (-NH3) carboxyl group (-COOH) R-group Ala, Gly, Ile, Leu, Val 9.7 2.3 ---- Asn, Gln 9.0 2.1 ---- Met, Ser, Trp 9.3 2.3 ---- Phe 9.1 1.8 ---- Pro 10.6 2.0 ---- Thr 10.4 2.6 ---- Asp 9.8 2.1 3.9 Glu 9.7 2.2 4.2 His 9.2 1.8 6.0 Cys 10.8 1.8 8.3 Tyr 9.1 2.2 10.0 Lys 9.0 2.2 10.5 Arg 9.0 2.2 12.5 For the multiple choice questions, please select the BEST answer and bubble in on your scantron. SC1. (4 points) Adding 100 mL of 1 M NaOH to a solution of acetic acid (CH3COOH) will: A. Result in 0.1 fewer moles of CH3COOH B. Create 0.1 more moles of CH3COOH C. Result in 0.1 fewer moles of CH3COO- D. Result in 0.01 more moles of CH3COO- E. None of the above are correct SC2. (4 points) Which of the following molecular situations demonstrates hydrophobic interactions? A. The amino group of a dipeptide is attracted to the R-group of aspartate at intracellular conditions (pH=7). B. Nonpolar C—H bonds of one molecule cluster with polar O—H bonds of another molecule to decrease entropy of surrounding water molecules. C. Transient dipoles of two atoms are attracted to each other within a particular distance between the nuclei of the atoms. D. Nonpolar hydrocarbon chains of different molecules will cluster together in aqueous environments to minimize the number of ordered water molecules surrounding them. E. Entropy is decreased as nonpolar region of molecules experience attraction toward one another in aqueous environments. SC3. (3 points) Pick the strongest base: A. Ammonium ion (Ka= 5.8 * 10-10 ) B. Hydrogen carbonate ion (Ka= 4.7 * 10-11 ) C. Hydrogen phosphate ion (Ka= 4.2 * 10-13 ) D. Dihydrogen borate ion (Ka= 1.8 * 10-13 ) SC4. (3 points) What is the overall charge of histidine at pH=12? A. -2 B. -1 C. 0 D. +1 E. +2 SC5. (2 points) For the hydrolysis of ATP into ADP and inorganic phosphate (Pi), the ΔG’° is -13,700 J/mol. At standard biochemical conditions A. the production of ATP is favored B. the production of ADP is favored C. the reaction is endergonic D. there is no net flux of the reaction E. the products are at a higher energy state than the reactants SC6. (4 points) For the reaction in question 5, which value is closest to the observed ΔG at 37C if [ATP]= 0.1 M, [ADP]=5 M, and [Pi]=5 M? A. -43 kJ/mol B. -28 kJ/mol C. 0.5 kJ/mol D. 0.04 kJ/mol E. not enough information is provided to do this calculation SC7. (3 points) Imagine a planet in an alternate universe where the primary solvent of life is H2Ω. Ω has two pairs of lone electrons just like oxygen, but is much less electronegative. How will the freezing point of H2Ω compare to H2O? A. The freezing point will increase B. The freezing point will decrease C. The freezing point will stay the same D. Insufficient data to determine SC8. (3 points) How would the solubility of NaCl from earth behave when dissolved in H2Ω when compared to H2O? A. NaCl will be more soluble in H2Ω B. NaCl will be less soluble in H2Ω C. NaCl will be equally soluble in H2O as in H2Ω D. Insufficient data to determine SC9. (3 points) Which value is closest to the pH of a 0.2 M NaOH solution? A. 1.5 B. 3 C. 7.5 D. 10.5 E. 13 SC10. (3 points) The reaction A ßà B will go in the forward direction if: A. S is positive B. TS is positive and greater than H C. H is positive and S is positive D. A is more organized than B SC11. (3 points) If a reaction under standard conditions has a K’eq that is greater than 1, the reaction: A. is at equilibrium and the concentration of products equals the concentration of reactants. B. is favored to proceed in the reverse direction C. is favored to proceed in the forward direction D. is endergonic and will not proceed without an input of energy. A solution of Histidine was titrated with NaOH, and pH was measured as base was added. The results were plotted as shown in the graph below. For each of the statements in questions 9-11, identify the corresponding point in the titration by marking A, B, C, D, or E. (3 points each) SC112. Histidine’s amino group and R group are predominantly protonated (--NH3+ and --RH+) but the carboxyl group is totally deprotonated (--COO-). B SC13. Half of the amino groups of Histidine are ionized. E SC14. The pH is equal to the pKa of the carboxyl group of Histidine. A For the following questions, please fill in A on your scantron if the answer is true and B if the answer is false. (2 points each) SC15. T/F The pKa of the COOH group of the tripeptide AAA will be the same as the pKa of the COOH group of Alanine. SC16. T/F A reaction that is endothermic (positive change in enthalpy) may be exergonic or endergonic. SC17. T/F A reaction with a free energy change of +15 kJ/mol can occur spontaneously by being coupled with a reaction that has a free energy change of -5 kJ/mol. SC18. T/F The peptide GNRAVY absorbs more UV light at 280 nm than the peptide ALWMQR. SC19. T/F Electrons are shared between atoms in a hydrogen bond. SC20. T/F Even for very large molecules the Van der Waals attraction between the molecules will not be as strong as a single hydrogen bond. SC21. T/F: Hydrogen bonds are unique to water SC22. T/F: Equilibrium is reached at the midpoint of a titration of a weak acid, when the [HA] = [A-]. SC23. T/F: A large Keq means that the reaction tends to proceed until reactants are almost completely converted to products. SC24. T/F: The standard transformed free-energy change of a chemical reaction (G) is an alternative mathematical way of expressing it’s Keq SC25. T/F: Hydrogen bonds can impart directionality on the interacting molecules. SC26. T/F: A single water molecule can form hydrogen bonds with three other water molecules. SC27. T/F: The criteria for whether a reaction will be spontaneous or not is a negative value for that reaction’s G’o. SC28. T/F: A reaction that starts farther away from equilibrium will have a larger magnitude G than one that starts closer to equilibrium. SC29. T/F: Van Der Waals interactions are very weak and thus do not contribute greatly to interactions between biomolecules. SC30. T/F: Hydrophobic interactions are driven by enthalpy (H) changes because water is more organized when it is in contact with a hydrophobic surface. Written response: For full points, please show all your work. 1. Carnosine is a dipeptide that acts as a buffer to maintain intramuscular pH. During intense exercise, hydrogen ions are produced leading to a drop in intramuscular pH and the protonation of the carnosine zwitterion on its imidazole ring. The ionization reaction is: carnosine zwitterion + hydrogen ion --> carnosine cation + water a. (4 points) What two amino acids residues are linked together in carnosine? Write their names and one letter codes. b. (3 points) Draw the cationic form of carnosine that would predominate after intense exercise. c. (5 points) Given the following information, calculate the concentration of the carnosine zwitterion and cation present before intense exercise. i. Given: 1. The baseline (pre-exercise) pH= 7.17 2. Total intramuscular carnosine concentration = 5 mM 3. Carnosine zwitterion pKa= 6.84 3. (12 points) How many moles of NaH2PO4 and Na2HPO4 would you combine to make 3 Liters of 0.2 M phosphate buffer at pH=7? The pKa of dihydrogen phosphate is 6.86. Report your final answer in moles and round to two places past the decimal point. Show all of your work for full credit. NaH2PO4: Na2HPO4: Scantron score Written answer 1 [Show More]
Last updated: 9 months ago
Preview 1 out of 7 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 16, 2020
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Sep 16, 2020
Downloads
1
Views
155