Chemical Equations and Reaction Stoichiometry Test Bank Rated A
Document Content and Description Below
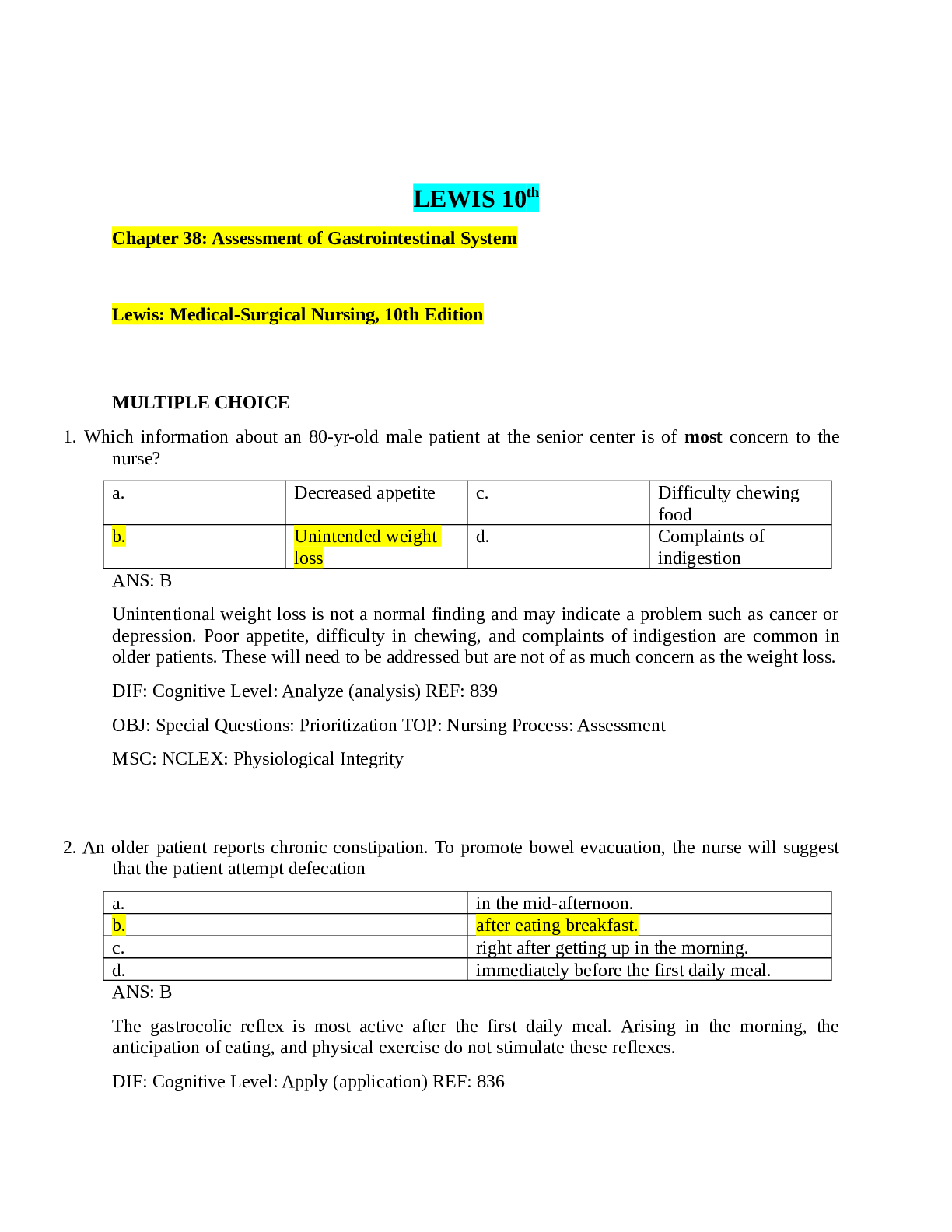
MULTIPLE CHOICE 1. Which of the following is not a consequence of the Law of Conservation of Matter? a. It provides a basis for balancing chemical equations. b. It means that there will be no obser... vable change in the quantity of matter during a chemical reaction. c. As a result, there will be the same number of moles on both the reactant and the product side of a balanced equation. d. It can be stated as "matter is neither created nor destroyed during a chemical reaction." e. All of these are a consequence of the Law of Conservation of Mass. ANS: C OBJ: Understand the implications of the Law of Conservation of Matter. TOP: Chemical Equations 2. Balancing a chemical equation so that it obeys the law of conservation of matter requires: a. Adjusting the coefficients in front of the formulas so there are the same number and type of atom on both sides of the equation. b. Making sure the reactants and products are in the same phase. c. Keeping the total charge the same on both sides of the equation. d. Changing the formulas of the products and reactants. e. Keeping the same number of molecules on both sides of the equation. ANS: A OBJ: Understand the implications of the Law of Conservation of Matter. TOP: Chemical Equations 3. What scientific law requires that subscripts in formulas should never be changed while balancing a chemical equation? a. Law of Multiple Proportions b. Law of Definite Proportions c. Law of Conservation of Matter d. Law of Conservation of Matter and Energy e. Law of Conservation of Energy ANS: B OBJ: Understand the implications of the Law of Definite Proportions. TOP: Chemical Equations 4. Balance the following equation with the smallest whole number coefficients. What is the coefficient for O2 in the balanced equation? C4H10 + O2 CO2 + H2O a. 9 b. 5 c. 15 d. 6 e. 13 Whitten 10e Test Bank ANS: E OBJ: Balance a chemical equation. TOP: Chemical Equations 5. Balance the following equation with the smallest whole number coefficients. What is the coefficient for O2 in the balanced equation? C4H9SO + O2 CO2 + SO2 + H2O a. 54 b. 29 c. 23 d. 32 e. 27 ANS: E DIF: Harder Question OBJ: Balance a chemical equation. TOP: Chemical Equations 6. What is the coefficient for carbon dioxide when the following equation showing the combustion of isopropyl alcohol is balanced with the smallest whole number coefficients? C3H8O + O2 CO2 + H2O a. 3 b. 6 c. 13 d. 4 e. 1 ANS: B OBJ: Balance a chemical equation. TOP: Chemical Equations 7. Balance the following equation with the smallest whole number coefficients. What is the coefficient for H2O in the balanced equation? Al(OH)3 + HCl AlCl3 + H2O a. 1 b. 2 c. 3 d. 14 e. 5 ANS: C OBJ: Balance a chemical equation. TOP: Chemical Equations Whitten 10e Test Bank 8. Balance the following equation with the smallest whole number coefficients. What is the coefficient for H2O in the balanced equation? LiBF4 + H2O H3BO3 + HF + LiF a. 3 b. 2 c. 5 d. 6 e. 8 ANS: A OBJ: Balance a chemical equation. TOP: Chemical Equations 9. What is the coefficient for HBr when the following equation is balanced with the smallest whole number coefficients? Br2 + H2O HBr + HBrO3 a. 5 b. 7 c. 8 d. 3 e. 6 ANS: A OBJ: Balance a chemical equation. TOP: Chemical Equations 10. Balance the following equation with the smallest whole number coefficients. What is the coefficient for NH3 in the balanced equation? Fe(NO3)3 + NH3 + H2O Fe(OH)3 + NH4NO3 a. 1 b. 3 c. 2 d. 6 e. 4 ANS: B OBJ: Balance a chemical equation. TOP: Chemical Equations 11. Elemental phosphorus is produced from calcium phosphate in the following reaction. What is the coefficient for C when this equation is balanced with the smallest whole number coefficients? Ca3(PO4)2 + SiO2 + C P4 + CO + CaSiO3 a. 10 b. 3 c. 1 d. 6 e. 4 ANS: A OBJ: Balance a chemical equation. TOP: Chemical Equations [Show More]
Last updated: 1 year ago
Preview 1 out of 38 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 03, 2021
Number of pages
38
Written in
Additional information
This document has been written for:
Uploaded
Sep 03, 2021
Downloads
0
Views
66





.png)
.png)
.png)