Physics > QUESTIONS & ANSWERS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity (All)
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. All Done. 100%
Document Content and Description Below
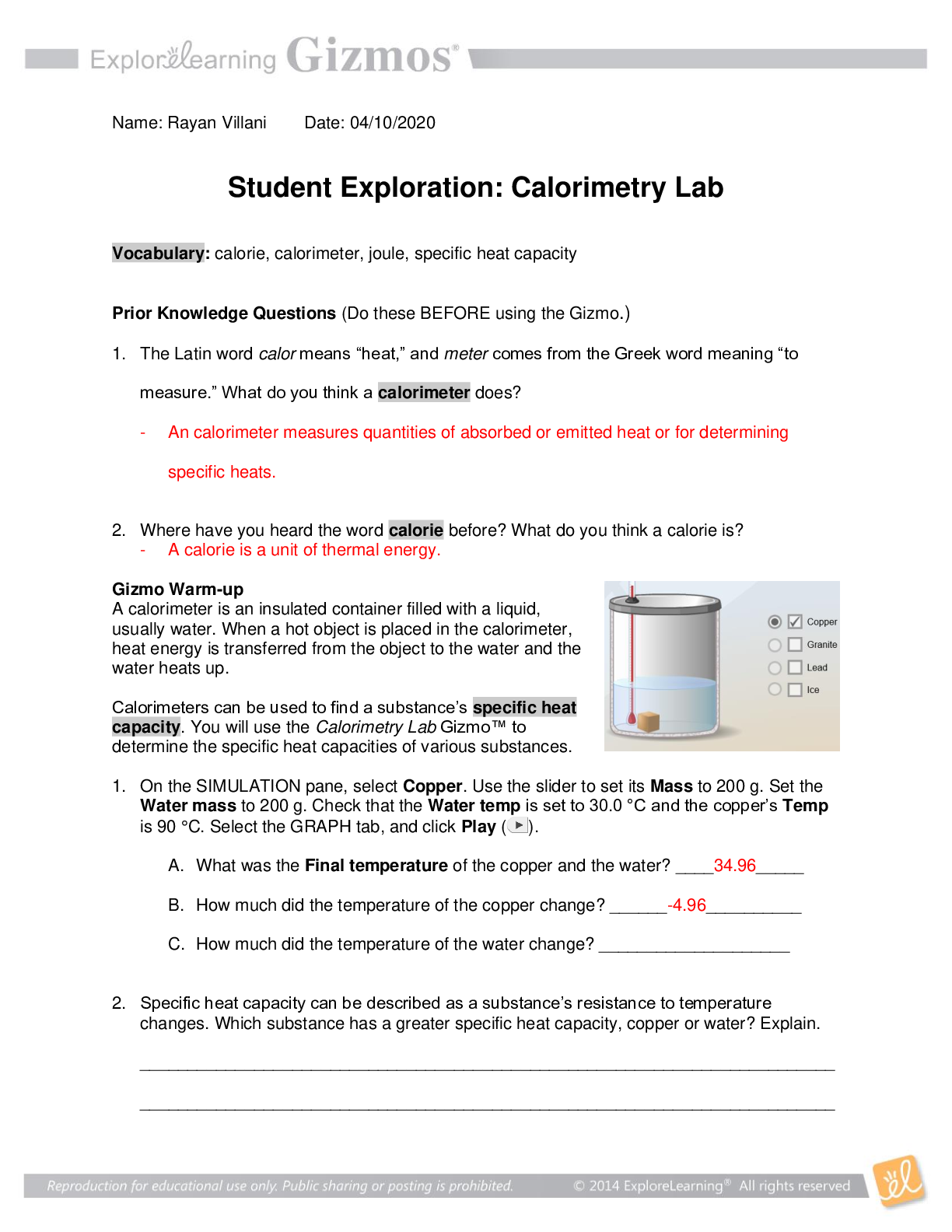
Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “heat,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter doe... s? 2. Where have you heard the word calorie before? What do you think a calorie is? Gizmo Warm-up A calorimeter is an insulated container filled with a liquid, usually water. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat capacity. You will use the Calorimetry Lab Gizmo™ to determine the specific heat capacities of various substances. 1. On the SIMULATION pane, select Copper. Use the slider to set its Mass to 200 g. Set the Water mass to 200 g. Check that the Water temp is set to 30.0 °C and the copper’s Temp is 90 °C. Select the GRAPH tab, and click Play ( ). A. What was the Final temperature of the copper and the water? B. How much did the temperature of the copper change? C. How much did the temperature of the water change? 2. Specific heat capacity can be described as a substance’s resistance to temperature changes. Which substance has a greater specific heat capacity, copper or water? Explain. Activity A: Heat transfer Get the Gizmo ready: ● Click Reset ( ). Question: What factors determine how heat energy transfers between objects? 1. Predict: In the Gizmo warm-up, you saw how 200 g of 90 °C copper transfers heat to 200 g of 30.0 °C water. A. How do you think increasing the water’s mass would affect the final temperature? B. How do you think decreasing the copper’s mass would affect the final temperature? C. How do you think increasing or decreasing the copper’s initial temperature would affect the final temperature? 2. Collect data: Use the Gizmo to determine the final temperature for each set-up listed below. Record your results in the tables. In the first table, you experiment with changing the water’s mass. In the second table, you change the copper’s mass. In the third table, you change the initial temperature of the copper. The first row of each table has been completed for you. Copper Water Final Temp. (°C) Initial Temp. (°C) Mass (g) Initial Temp. (°C) Mass (g) 3. 90 °C 200 g 30.0 °C 34.96 °C 90 °C 200 g 30.0 °C 30.54C 4. 90 °C 30.0 °C 200 g 34.96 °C 90 °C 30.0 °C 200 g 30.54C 5. 200 g 30.0 °C 200 g 34.96 °C 200 g 30.0 °C 200 g 35.79C 200 g 30.0 °C 200 g 31.65C 3. Analyze: For each factor listed in the chart below, explain how the final temperature was changed and why you think that change occurred. A. What was the effect of increasing the water’s mass? B. What was the effect of decreasing the copper’s mass? C. What was the effect of changing the initial temperature of the copper? 4. Draw conclusions: The amount that the water’s temperature increases depends on the mass of the water and the amount of heat energy in the copper. A. How does changing the initial mass of the copper affect how much heat energy it has? B. How does changing the initial temperature of the copper affect how much heat energy it has? 5. Apply: Many gyms and health clubs have steam saunas, which are small steam-filled rooms. Traditionally, steam saunas have a container of heated rocks. A small ladle of water is poured on the rocks in order to make the steam. A. Use what you have learned so far about heat transfer to explain how hot rocks can be used to make steam? B. Why do you think only a small ladle-full of water is poured on the rocks at one time? Activity B: Specific heat Get the Gizmo ready: ● Click Reset. ● Deselect Copper, and select Granite. Question: How can you compare the specific heat capacities of various substances? 1. Explain: How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the Gizmo? 2. Predict: Which substance do you think will have the highest specific heat capacity? Why? 3. Experiment: Use the Gizmo to determine the final temperature for each set-up listed below. Record your results in the table. The first row has been completed for you. Substance Substance initial temp. (°C) Substance mass Water initial temp. (°C) Water mass Final temp. (°C) Copper 90 °C 200 g 30.0 °C 200 g 34.96 °C Granite 90 °C 200 g 30.0 °C 200 g 39.59C Lead 90 °C 200 g 30.0 °C 200 g 40.83C 4. Analyze: Of the three substances, which caused the largest temperature change in the water? What does this indicate about its relative specific heat capacity? 5. Interpret: Remember that specific heat capacity is a measure of a substance’s resistance to temperature change. The more resistant a substance is to temperature change, the higher is its specific heat capacity. Rank the three substances in order of their specific heat capacities, from highest to lowest. 6. Predict: How do you think the specific heat capacity of ice will compare to that of copper, granite, and lead? 7. Experiment: Deselect Lead, and select Ice. Use the default values for Temp (-30 °C) and Mass (50 g). Set the Water temp to 60 °C and the Water mass to 200 g. Click Play. A. What was the final temperature? B. What do you think is happening when the ice line on the graph is at 0 °C for a long period of time? Why do you think the line disappears after that? C. How much of a temperature change did the water experience? D. How does this change in the water’s temperature compare to the change caused by the other substances you tested? 8. Extend your thinking: A lot of energy is needed to heat a substance with a high specific heat capacity. However, even more energy is needed to cause a phase change (such as the melting of ice). Click Reset. Set the ice’s Temp to -100 °C and its Mass to 50 g. Set the Water temp to 50 °C and Water mass to 200 g. Click Play. A. What was the final temperature? B. Do you think all the ice melted? Explain. C. Look at the GRAPH. The graph shows two separate stages: the heating of the ice and then the melting of the ice. How much did the water’s temperature change while the ice was heating? How much did it change while the ice was melting? D. How did this experiment demonstrate ice’s high specific heat capacity? Activity C: Calculating specific heat Get the Gizmo ready: ● Click Reset. Introduction: The specific heat capacity of a substance is the amount of energy needed to change the temperature of that substance by 1 °C. Specific heat capacity can be calculated using the following equation: q = mc∆T In the equation q represents the amount of heat energy gained or lost (in joules), m is the mass of the substance (in grams), c is the specific heat capacity of the substance (in J/g °C), and ∆T is the temperature change of the substance (in °C). Goal: Calculate the specific heat capacities of copper, granite, lead, and ice. 1. Solve: A. Water has a known specific heat capacity of 4.184 J/g °C. Use the specific heat equation to find out how much heat energy the water gained (q). B. Assume that the heat energy gained by the water is equal to the heat energy lost by the aluminum. Use the specific heat equation to solve for the specific heat of aluminum. (Hint: Because heat energy is lost, the value of q is negative.) Aluminum’s accepted specific heat value is 0.900 J/g °C. Use this value to check your work. 2. Calculate: Use the Gizmo to mix 200 g of copper at 100 °C with 1,000 g of water at 20 °C. A. What is the final temperature? B. Calculate the temperature change of each substance by subtracting the initial temperature from the final temperature. ∆Twater: ∆Tcopper: C. How much heat energy (q) did the water gain? D. Now solve for the specific heat (c) of copper: 3. Calculate: Use the Gizmo to mix 200 g of granite at 100 °C with 1,000 g of water at 20 °C. A. What is the final temperature? B. Calculate the temperature change of each substance by subtracting the initial temperature from the final temperature. ∆Twater: ∆Tgranite: C. How much heat energy (q) did the water gain? D. Now solve for the specific heat (c) of granite: . E. Repeat steps A through D to find the specific heat (c) of lead: 4. Challenge: Use the specific heat capacity that you calculated for granite to determine how many grams of granite at the initial temperature of 80 °C must mix with 3,000 g of water at the initial temperature of 20 °C to result in a final system temperature of 20.45 °C. (Hint: Start by calculating how much heat energy is needed to change the water’s temperature by 0.45 °C). Show your work. Use the Gizmo to check your answer. 5. Extend your thinking: In addition to calculating specific heat capacities, some calorimeters can be used to determine how much energy is in food. The energy in food is usually expressed in calories or kilocalories (Calories). A calorie is the amount of energy needed to change the temperature of 1 g of water by 1 C. There are 1,000 calories in a Calorie. A. How many joules are in 1 calorie? (The specific heat of water is 4.184 J/g °C.) B. Suppose a snack bar is burned in a calorimeter and heats 2,000 g water by 20 °C. How much heat energy was released? (Hint: Use the specific heat equation.) Give your answer in both joules and calories. C. How many kilocalories (Calories) does the snack bar contain? [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
Reviews( 0 )
Recommended For You
Physics> QUESTIONS & ANSWERS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. All Done. 100% (All)

Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. All Done. 100%
Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “heat,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter does? 2. Wh...
By Tutor Frankline , Uploaded: Jul 19, 2021
$12
Chemistry> QUESTIONS & ANSWERS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. (All)
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity.
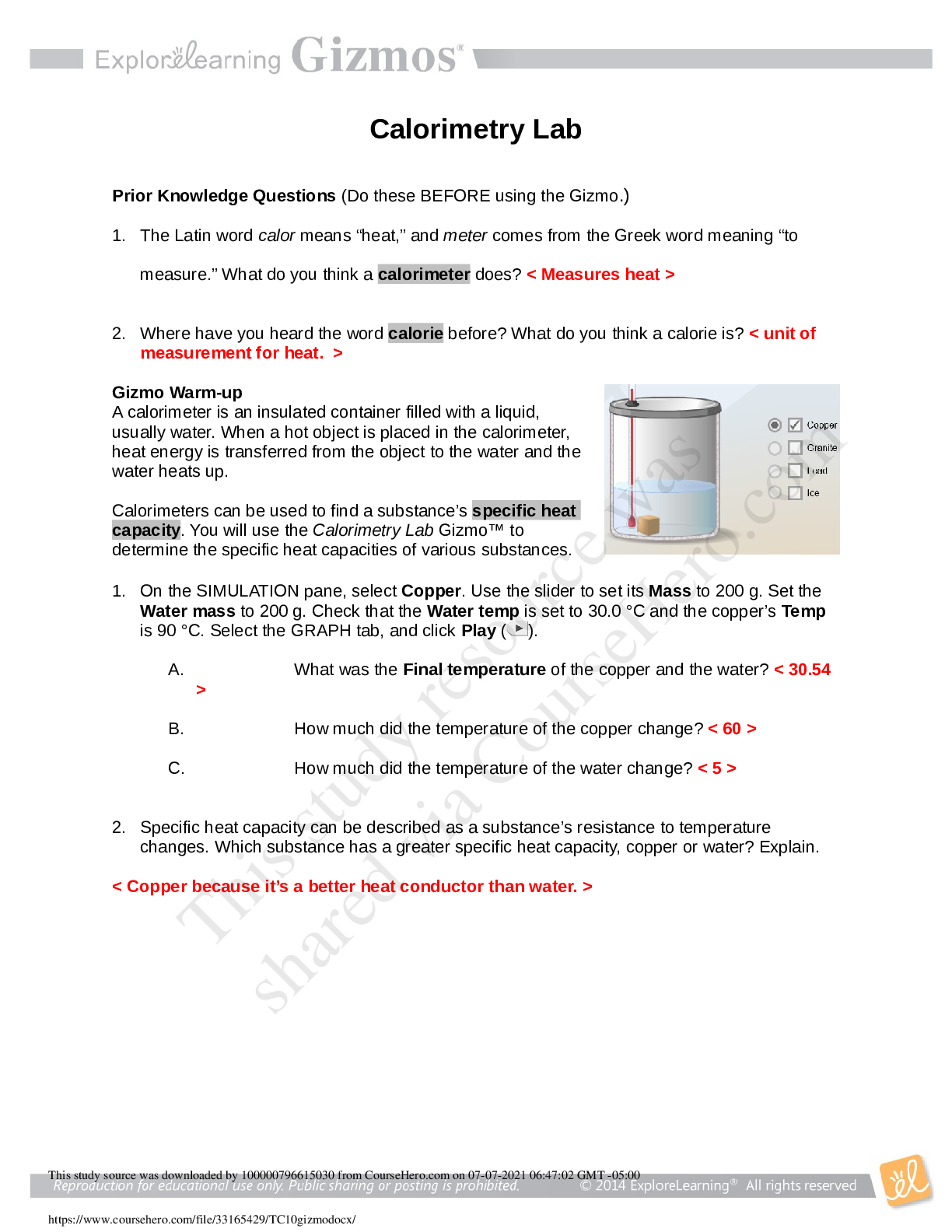
1. The Latin word calor means “heat,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter does? < Measures heat > 2. Where have you heard the word calorie befo...
By destinyd , Uploaded: May 11, 2021
$5.5
*NURSING> QUESTIONS & ANSWERS > NCSBN TEST BANK for the NCLEX-RN & NCLEX-PN. Contains More than 2000 Q&A Plus Review and Rationale in 517 PAGES. (All)

NCSBN TEST BANK for the NCLEX-RN & NCLEX-PN. Contains More than 2000 Q&A Plus Review and Rationale in 517 PAGES.
NCSBN TEST BANK -for the NCLEX-RN & NCLEX-PN. Updated 2022/2023. Contains More than 2000 Q&A Plus Review and Rationale in 517 PAGES. (All Testable Questions for NCLEX-RN & NCLEX-PN)
By Expert1 , Uploaded: Jul 28, 2020
$20
Business> QUESTIONS & ANSWERS > CLM 031 EXAM (All)

CLM 031 EXAM
CLM 031 = 100% Question 1: 5b Select the statement that is correct concerning performance work statement (PWS) requirements: - All answers are correct. - PWS should describe requirements necessary...
By Book Worm, Certified , Uploaded: Nov 03, 2022
$5
*NURSING> QUESTIONS & ANSWERS > PHIL 347 Week 6 Checkpoint Quiz. Score 100/100 (All)

PHIL 347 Week 6 Checkpoint Quiz. Score 100/100
Question: What are the three fundamental reasoning strategies listed in the text? Question: What is comparative reasoning? On what skill is it based? Question: We learned four tests for evaluating...
By Amanda Rosales , Uploaded: Mar 24, 2021
$7
Business> QUESTIONS & ANSWERS > BUSINESS 1007 (All)

BUSINESS 1007
BUSINESS 1007 07 Key 1. (p. 178) Managers utilize organizational resources such as employees, information, and equipment to accomplish goals. 2. (p. 178) The main job of managers today is to w...
By Kirsch , Uploaded: Oct 19, 2019
$6
Anthropology> QUESTIONS & ANSWERS > KOR 352 FA19 101 week 8 Quiz. Already Graded A (All)

KOR 352 FA19 101 week 8 Quiz. Already Graded A
KOR 352 FA19 101: Week 8 Quiz Question 1 (0.25 points) Which of the following is not true of Kim and Finch’s observations during their field research in South Korea from 1997 to 2000? Question 1 o...
By Kirsch , Uploaded: Oct 17, 2019
$9
E-Commerce> QUESTIONS & ANSWERS > ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score (All)
ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score
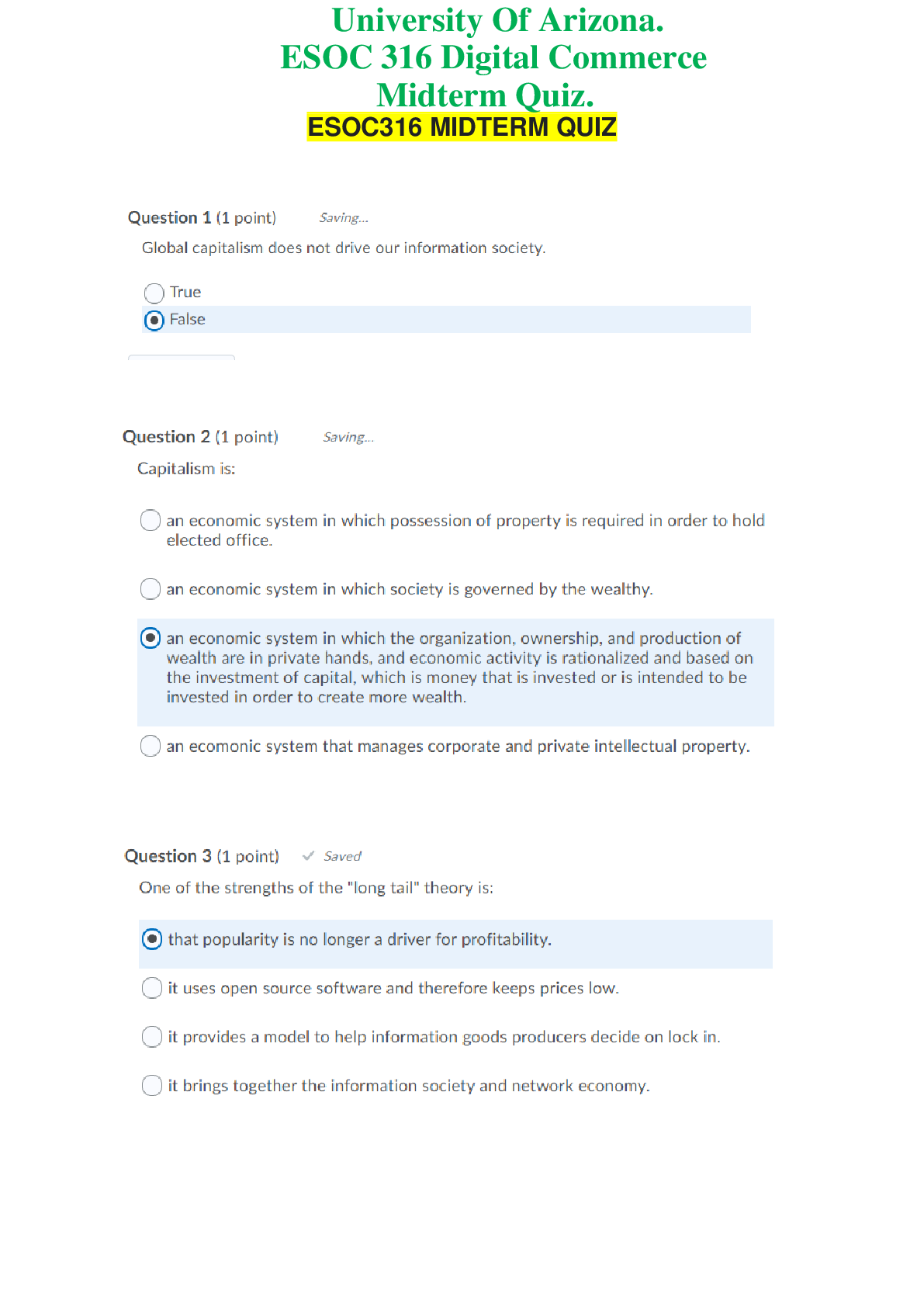
ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score ESOC316 MIDTERM QUIZQuestion 6 (1 point) Saved Information has several properties that make information goods...
By Kirsch , Uploaded: Oct 15, 2019
$9.5
Marketing> QUESTIONS & ANSWERS > Marketing Management Chapter 2 to Chapter 10 Q&A (All)

Marketing Management Chapter 2 to Chapter 10 Q&A
Chapter 2 to Chapter 10 Chapter 2: Developing Marketing Strategies and Plans GENERAL CONCEPT QUESTIONS Multiple Choice 66 Chapter 1: Marketing: Managing Profitable Customer Relationships...
By Kirsch , Uploaded: Oct 14, 2019
$10
Marketing> QUESTIONS & ANSWERS > MKT 530 Customer Relationship Management. 155 Questions and Answers (All)

MKT 530 Customer Relationship Management. 155 Questions and Answers
MKT 530 All Questions and Answers MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) As the manager of an organization that is att...
By Kirsch , Uploaded: Oct 14, 2019
$10
Document information
Connected school, study & course
About the document
Uploaded On
Mar 05, 2021
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Mar 05, 2021
Downloads
0
Views
278






