Applied Science > EXAM > BTEC Applied Science Unit 1 Chemistry Exam 271 Questions with Answers,100% CORRECT (All)
BTEC Applied Science Unit 1 Chemistry Exam 271 Questions with Answers,100% CORRECT
Document Content and Description Below
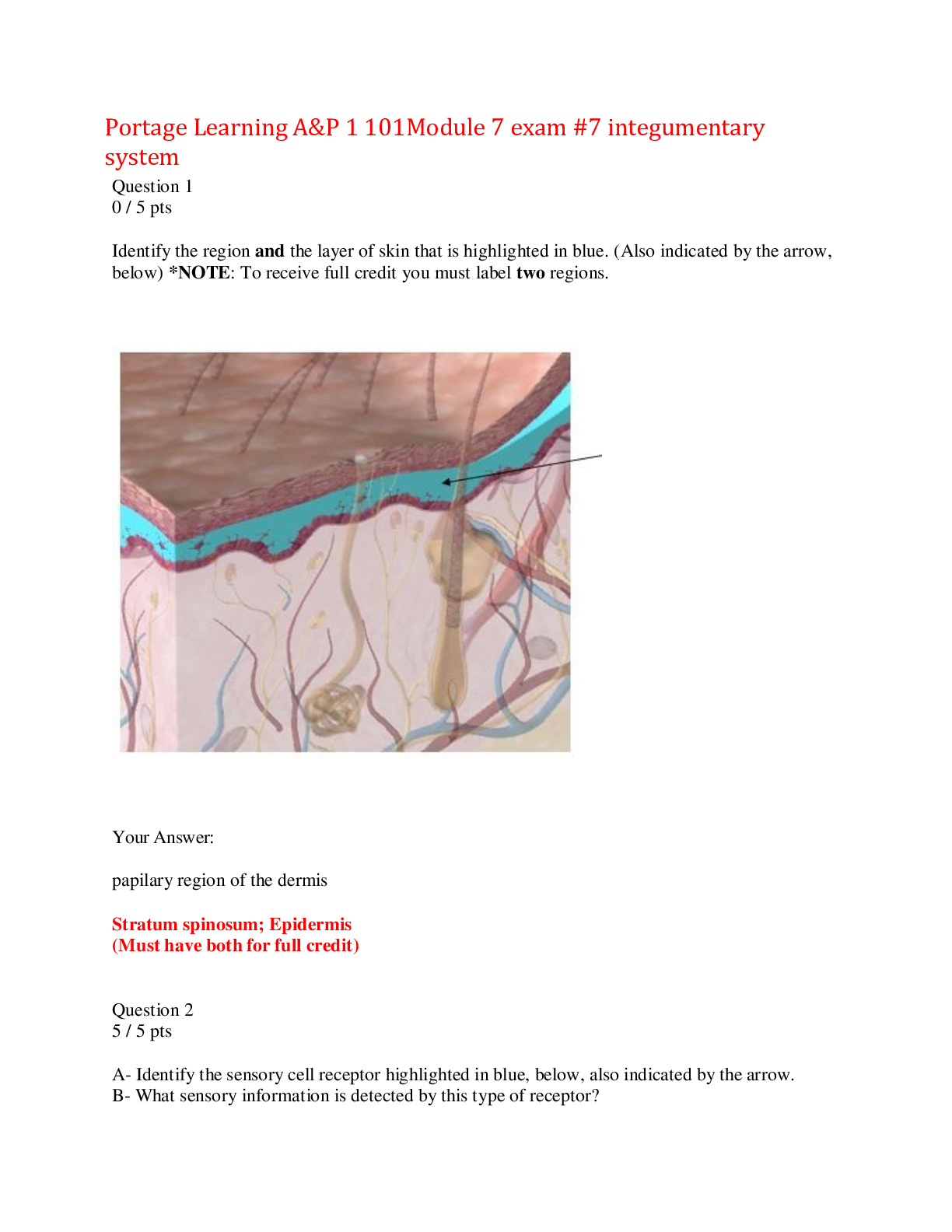
BTEC Applied Science Unit 1 Chemistry Exam 271 Questions with Answers How many electrons can an s subshell hold? - CORRECT ANSWER 2 How many electrons can a p subshell hold? - CORRECT ANSWER 6... How many electrons can a d subshell hold? - CORRECT ANSWER 10 Which subshells are available in the first energy level? - CORRECT ANSWER s Which subshells are available in the second energy level? - CORRECT ANSWER s and p Which subshells are available in the third energy level? - CORRECT ANSWER s, p and d What is Hund's rule? - CORRECT ANSWER Orbitals must all be singly filled before they can be doubly occupied Which elements do not fill the 4s subshell before the 3d subshell? - CORRECT ANSWER Copper and chromium Define the term ionic bond - CORRECT ANSWER The electrostatic attraction between oppositely charged ions What is the charge of an ion from group 1? - CORRECT ANSWER +1 What is the charge of an ion from group 2? - CORRECT ANSWER +2 What is the charge of an ion from group 6? - CORRECT ANSWER -2 What is the charge of an ion from group 7? - CORRECT ANSWER -1 Explain how atoms of sodium react with atoms of chlorine - CORRECT ANSWER Na loses its 2s1 electron gaining a +ve charge. Cl gains an electron in the 3p subshell gaining a -ve charge. The opposite charges attract to form NaCl Why do ionic bonds have such high melting points? - CORRECT ANSWER Each +ve ion is surrounded by 6 -ve ions and vice versa. Strong electrostatic attraction in every direction. Requires a large amount of energy to break State two factors that affect the strength of an ionic bond - CORRECT ANSWER Size of ion and charge on ion When can ionic substances conduct electricity? - CORRECT ANSWER When molten or in aqueous solution Describe the properties of ionic compounds - CORRECT ANSWER Conduct electricity when molten or aqueous solution High melting/boiling points Usually soluble in water Define the term covalent bond - CORRECT ANSWER A shared pair of electrons Which metals lose electrons from the 4s subshell before the 3d subshell? - CORRECT ANSWER Transition metals Why do metals have such high melting points? - CORRECT ANSWER Strong force of attraction between positive ions and delocalised electrons. This requires a large amount of energy to overcome. State the two factors that affect the strength of metallic bonding - CORRECT ANSWER Size of ion Charge on ion Explain how the charge on metal ions affects the strength of the metallic bond - CORRECT ANSWER The larger the +ve charge the greater the attraction between the nucleus and the delocalised electrons Explain how the size of the metal ions affects the strength of the metallic bond - CORRECT ANSWER The smaller the +ve ion the closer the nucleus is to the delocalised electrons creating a greater attraction Explain why metals conduct electricity - CORRECT ANSWER The delocalised electrons 'carry' charge. Current flows because of this. Explain why metals conduct heat - CORRECT ANSWER Particles are paced tightly so kinetic energy is passed from ion to ion. The delocalised electrons also enable heat to be passed. Explain why metals are ductile and malleable - CORRECT ANSWER The lattice structure allows layers of metal ions to slide over each other without disrupting bonding Name the 3 forces between molecules - CORRECT ANSWER Van der Waals Permanent dipole-dipole Hydrogen bonds Order the 3 forces between molecules in order of strongest to weakest - CORRECT ANSWER Hydrogen bonds Permanent dipole-dipole Van der Waals How are Van der Waal's forces formed? - CORRECT ANSWER Electrons move to one side, caused temporary dipole. This induces a temporary dipole in neighbouring molecules. Attraction occurs between oppositely charged dipoles In what molecules do Van der Waal's forces exist? - CORRECT ANSWER Non-polar molecules How are permanent dipole-dipole forces formed? - CORRECT ANSWER Permanent dipole in one molecule attracts oppositely charged permanent dipole in neighbouring molecule In which molecules do permanent dipole-dipole forces exist? - CORRECT ANSWER Polar molecules Which elements must be present for hydrogen bonds to exist? - CORRECT ANSWER Hydrogen and either nitrogen, oxygen or fluorine What is meant by the term displacement? - CORRECT ANSWER When a more reactive element takes the place of a less reactive element in a compound State the equation for determining moles - CORRECT ANSWER Moles = mass ÷ relative atomic mass (molar mass) Define the term Avogadro's Constant - CORRECT ANSWER The number of atoms in a mole of a given substance. Quoted as 6.02x10^23 Define the term relative atomic mass - CORRECT ANSWER The average mass of an atom of an element relative to 1/12th the relative atomic mass of Carbon12 Define the term relative molecular mass - CORRECT ANSWER The average mass of a molecule relative to 1/12th the relative atomic mass of Carbon12 What does this number represent? 6.02x10^23 - CORRECT ANSWER The number of particles in a mole. Commonly called Avogadro's Constant What is the equation for calculating % yield? - CORRECT ANSWER % yield = (actual yield ÷ theoretical yield) x 100 What groups are included in the 's' block of the periodic table? - CORRECT ANSWER Groups 1 and 2 What part of the periodic table is known as the 'd' block? - CORRECT ANSWER Transition metals Which groups are in the 'p' block of the periodic table? - CORRECT ANSWER 3, 4, 5, 6 and 7 What is a group on the periodic table? - CORRECT ANSWER A vertical column What is a period on the periodic table? - CORRECT ANSWER A horizontal row Define the term first ionisation energy - CORRECT ANSWER The energy required to remove the outermost electron from one mole of gaseous atoms to produce one mole of gaseous +1 ions Define the term atomic radius - CORRECT ANSWER The distance between the nucleus of an atom and the outermost electron Define the term electronegativity - CORRECT ANSWER A measure of how well an atom attracts a bonding pair of electrons in a covalent bond Define the term malleability - CORRECT ANSWER How easily a material can be hammered into shape Define the term ductility - CORRECT ANSWER How easily a material can be drawn into wires Describe the trend in atomic radius down any group - CORRECT ANSWER Atomic radius increases Explain the trend in atomic radius down any group - CORRECT ANSWER Higher energy levels are filled. The orbitals in higher energy levels are further from the nucleus Describe the trend in first ionisation energy down groups 1 and 2 - CORRECT ANSWER First ionisation energy decreases Explain the trend in first ionisation energy down groups 1 and 2 - CORRECT ANSWER Increased electron shielding Greater atomic radius Smaller attraction to +ve nucleus SO electron is easier to remove requiring less energy Describe the trend in melting points down groups 1 and 2 - CORRECT ANSWER Melting point decreases Explain the trend in melting points down groups 1 and 2 - CORRECT ANSWER Strength of metallic bond is weaker due to greater atomic radius decreasing attraction between +ve nucleus and delocalised electrons Describe the change in state as you go down group 7 - CORRECT ANSWER The trend is they become more solid (i.e. fluorine is a gas, bromine is a liquid and iodine is a solid) Describe the change in colour as you go down group 7 - CORRECT ANSWER They become darker as you go down the group Describe the trend in electronegativity down group 7 - CORRECT ANSWER Electronegativity decreases down the group Explain the trend in electronegativity down group 7 - CORRECT ANSWER Greater distance between nucleus and bonding electrons Greater electron shielding Decreases attraction between nucleus and electron Describe the trend in melting point down group 7 - CORRECT ANSWER Melting point increases down the group Explain the trend in melting point down group 7 - CORRECT ANSWER Atomic radius increases Stronger Van der Waal's forces More energy needed to overcome the intermolecular forces Describe the trend in atomic radius across a period - CORRECT ANSWER Atomic radius decreases Explain the trend in atomic radius across a period - CORRECT ANSWER Greater nuclear charge (more protons) Same number of electron shells Same amount of electron shielding Describe the trend in electronegativity across a period - CORRECT ANSWER Electronegativity increases across a period Explain the trend in electronegativity across a period - CORRECT ANSWER Same amount of electron shielding Greater number of protons More attraction between nucleus and bonding pair of electrons Describe the trend in melting point across a period - CORRECT ANSWER Melting point increases across the metals and then decreases throughout the non-metals Explain the trend in melting point across a period - CORRECT ANSWER Metallic bonding gets stronger across the period. All other intermolecular forces are weaker than this and therefore easier to break Why does the group 3 element have a lower first ionisation energy than the group 2 element - CORRECT ANSWER Electron taken from p subshell rather than s subshell so is further from the nucleus. Less energy is needed to remove the electron. Why does the group 6 element have a lower first ionisation energy than the group 5 element - CORRECT ANSWER Electron is taken from a paired orbital rather than a singly occupied orbital. Electron repulsion between the pair reduces the energy needed. Define the term displacement - CORRECT ANSWER When a more reactive element takes the place of a less reactive element in a molecule Describe the trend in reactivity down group 1 - CORRECT ANSWER They become more reactive down the group Define the term reduction - CORRECT ANSWER Reduction is gain of electrons Define the term oxidation - CORRECT ANSWER Oxidation is loss of electrons Define the term reducing agent - CORRECT ANSWER A reducing agent is something that loses electrons Define the term oxidising agent - CORRECT ANSWER An oxidising agent is something that gains electrons What oxidation state do group 1 metals have? - CORRECT ANSWER +1 What oxidation state to group 2 metals have? - CORRECT ANSWER +2 What oxidation state do group 6 elements have? - CORRECT ANSWER -2 What oxidation state do group 7 elements have? - CORRECT ANSWER -1 What is the overall oxidation state of a molecule? - CORRECT ANSWER 0 What is the overall oxidation state of a simple ion? - CORRECT ANSWER The charge on the ion What is the overall oxidation state of a molecular ion? - CORRECT ANSWER The charge on the ion What is the most common oxidation state of oxygen (with the exception of peroxides)? - CORRECT ANSWER -2 What is the overall oxidation state of an element? - CORRECT ANSWER 0 In a compound containing only two elements which will have the negative oxidation state? - CORRECT ANSWER The more electronegative element What is the oxidation state of hydrogen (except for in hydrides)? - CORRECT ANSWER +1 Is an atom oxidised or reduced if its oxidation number decreases? - CORRECT ANSWER Reduced Describe the function of the cytoplasm - CORRECT ANSWER Where all of the chemical reactions occur Describe the function of the vesicles - CORRECT ANSWER Transport materials in the cell and out of the cell Describe the function of the nucleolus - CORRECT ANSWER Produces ribosomes and RNA Describe the function of the cell wall - CORRECT ANSWER Provides rigidity and protection to the cell Describe the function of the chloroplasts - CORRECT ANSWER Site of photosynthesis as they contain chlorophyll Describe the function of the plasmodesmata - CORRECT ANSWER A channel through the cell wall the allows transport of materials from one cell to another Describe the function of the amyoplasts - CORRECT ANSWER Stores starch and converts starch back into glucose when the plant needs energy Describe the function of the vacuole - CORRECT ANSWER Stores water and chemicals for cell use. Also maintains turgor of cell Describe the function of the tonoplasts - CORRECT ANSWER Membrane the surrounds the vacuole. Protects the vacuole, isolates it from harmful substances and controls water flow in and out of the vacuole Why are gram positive bacteria more susceptible to antibiotics? - CORRECT ANSWER Permeable cell wall whereas gram negative bacteria has a semi-permeable cell wall Describe the adaptations found in a palisade cell - CORRECT ANSWER Closely packed together Chloroplasts Large vacuole Explain why the palisade cell has a large amount of chloroplasts - CORRECT ANSWER To absorb lots of light for photosynthesis Explain why palisade cells are closely packed together - CORRECT ANSWER To form a continuous layer in the leaf Explain why palisade cells have a large vacuole - CORRECT ANSWER To maintain turgor (pushes against the cell wall to maintain rigid shape) Describe the adaptations of a root hair cell - CORRECT ANSWER Root hair shape Large vacuole Thin cellulose walls Explain why root hair cells have an elongated section known as the root hair - CORRECT ANSWER Increased surface area for maximum movement of water into the cell Explain why root hair cells have a large vacuole - CORRECT ANSWER Contains cell sap with low water potential to encourage water into the cell Explain why root hair cells have thin cellulose walls - CORRECT ANSWER Encourage movement of water and minerals into the cell Describe the adaptations of a sperm cell - CORRECT ANSWER Undulipodium Lots of mitochondria Shaped head containing acrosome Haploid nucleus Explain why a sperm cell has an undulipodium - CORRECT ANSWER To allow the sperm to move to the egg Explain why sperm cells contain large numbers of mitochondria - CORRECT ANSWER To produce the large amounts of energy needed for movement Explain why sperm cells have a shaped head - CORRECT ANSWER To push through the protective layer of the egg cell Explain why sperm cells contain acrosome in the head - CORRECT ANSWER To digest the zona pellucida to allow entry into the egg cell Explain why sperm cells have a haploid nucleus - CORRECT ANSWER Contains half the genetic material. Describe the adaptations of egg cells - CORRECT ANSWER Contains a haploid nucleus Protective outer layer (zona pellucida) Corona radiata Explain why egg cells have a haploid nucleus - CORRECT ANSWER Contains half the genetic material Explain why egg cells have a zona pellucida (protective layer) - CORRECT ANSWER Protects the cell and only allows one sperm cell to enter. Explain why egg cells have a corona radiata (two or three layers attached to the zona pellucida) - CORRECT ANSWER Contains all of the proteins needed to develop the fertilised cell Describe the adaptations of red blood cells - CORRECT ANSWER No nucleus (mammals only) Biconcave shape Haemoglobin Flexible Explain why red blood cells don't contain a nucleus - CORRECT ANSWER Allows for more oxygen to be carried in the cell Explain why red blood cells have a biconcave shape - CORRECT ANSWER Increased surface area for gas exchange Explain why red blood cells contain haemoglobin - CORRECT ANSWER To bind with the oxygen in order for it to be carried around the body Explain why red blood cells are flexible - CORRECT ANSWER To fit through the tiny blood vessels such as capillaries Describe the adaptations of white blood cells - CORRECT ANSWER Multi-lobed nucleus Enzymes found within the lysosomes Explain why white blood cells have a multi-lobed nucleus - CORRECT ANSWER To enable the cell to squeeze through tiny gaps when travelling to the site of infection Explain why the lysosomes found within the cytoplasm of white blood cells contain enzymes - CORRECT ANSWER To digest engulfed pathogens Name the 3 different types of epithelial tissue - CORRECT ANSWER Squamous epithelial tissue Columnar epithelial tissue Endothelium tissue Describe squamous epithelial tissue - CORRECT ANSWER A lining tissue that is only one cell thick. Made from squamous cells that form a smooth, flat layer. Where can squamous epithelial tissue be found? - CORRECT ANSWER As it is a lining tissue it can be found inside alveoli How can squamous epithelial tissue be damaged by smoking? - CORRECT ANSWER Inflammation and scarring of tissue Tissue gets thicker and produces more mucus Diffusion pathway is increased What does COPD stand for? - CORRECT ANSWER Chronic Obstructive Pulmonary Disorder What symptoms can be displayed from COPD? - CORRECT ANSWER Breathlessness Persistent coughing Phlegm build up What conditions are considered part of COPD? - CORRECT ANSWER Emphysema Bronchitis Asthma Describe columnar epithelial tissue - CORRECT ANSWER Column shaped ciliated cells and goblet cells held in place by a membrane What is meant by the term ciliated cell? - CORRECT ANSWER A cell covered in cilia. Cilia are fine, hair like projections that 'sweep' away pathogens from the lungs How does smoking affect ciliated columnar tissue? - CORRECT ANSWER The cilia can break, lessening the 'sweeping action' overall. This leads to a build-up of mucus. What is the role of the goblet cell? - CORRECT ANSWER To produce mucus How do goblet cells and ciliated cells work together? - CORRECT ANSWER Goblet cells produce mucus that sticks to pathogens, whilst cilia 'sweep' away the mucus containing the pathogens How does smoking affect the alveoli? - CORRECT ANSWER Reduces the elasticity so pockets of air form that cannot be expelled Where is endothelial tissue found? - CORRECT ANSWER Lining the inside of blood vessels, lymphatic vessels and the heart Describe the structure of endothelial tissue - CORRECT ANSWER A layer of flattened cells, one layer thick What is atherosclerosis? - CORRECT ANSWER The process of white blood cells encouraging the deposition of fatty substances (cholesterol) below the endothelial lining What factors can increase the likelihood of atherosclerosis? - CORRECT ANSWER Smoking High blood pressure What conditions can atherosclerosis cause? - CORRECT ANSWER Angina TIA (mini strokes) Heart attack Aneurysm and haemorrhage Name the three types of muscle tissue - CORRECT ANSWER Skeletal Cardiac Smooth What type of control is exhibited in skeletal muscle? - CORRECT ANSWER Voluntary control What type of control is exhibited in cardiac muscle? - CORRECT ANSWER Involuntary control What type of control is exhibited in smooth muscle? - CORRECT ANSWER Involuntary control Where can skeletal muscle be found? - CORRECT ANSWER Attached to bones Where can cardiac muscle be found? - CORRECT ANSWER In the heart Where can smooth muscle be found? - CORRECT ANSWER In the walls of hollow organs such as the stomach and bladder Put the following in order of size from largest to smallest: fibre, muscle, filaments, myofibril, bundle of fibres - CORRECT ANSWER Muscle, bundle of fibres, fibre, myofibril, filaments Muscle fibres are many cells joined together. What organelles do these cells share? - CORRECT ANSWER Nuclei and cytoplasm, inside which are many mitochondria and specialised ER Why do muscle cells contain many mitochondria? - CORRECT ANSWER To provide large amounts of energy to the muscle for contractions Give the term used to describe the stripy bands seen within skeletal muscle under a microscope - CORRECT ANSWER Striations Myofibril is made up of dark and light bands. What are the names given to these dark and light bands? - CORRECT ANSWER Dark band = A-band Light band = I-band Which line is present in the middle of the A-band? - CORRECT ANSWER M line Which line is present in the middle of the I-band? - CORRECT ANSWER Z line What is the name given to the space between two Z-lines? - CORRECT ANSWER Sarcomere Myofibril is made up of alternating thick and thin filaments. What are the names of these filaments? - CORRECT ANSWER Thick = myosin filament Thin = actin filament What happens to the sarcomere during muscle contraction? - CORRECT ANSWER It shortens What are the two types of skeletal muscle? - CORRECT ANSWER Fast twitch and slow twitch Which sports are slow twitch muscle fibres best for? - CORRECT ANSWER Long distance running and cycling Fast twitch muscles can be further divided into two sub-categories, what are they? - CORRECT ANSWER Fast twitch oxidative muscles and fast twitch glycolytic muscles Which sports are fast twitch oxidative muscle fibres best for? - CORRECT ANSWER Mid-range sports such as1500m Which sports are fast twitch glycolytic muscle fibres best for? - CORRECT ANSWER Sprinting, short burst sports What type of exercise are slow twitch muscles designed for? - CORRECT ANSWER Aerobic exercise What type of exercise are fast twitch muscles designed for? - CORRECT ANSWER Anaerobic exercise In which type of muscle fibre is the speed of contraction slowest? - CORRECT ANSWER Slow twitch In which type of muscle fibre is the speed of contraction highest? - CORRECT ANSWER Fast glycolytic twitch Describe the characteristics of slow twitch muscle fibres - CORRECT ANSWER Less sarcoplasmic reticulum (specialised ER) More mitochondria More myoglobin A dense capillary network Describe the characteristics of fast oxidative muscle fibres - CORRECT ANSWER Similar to slow twitch with many mitochondria, myoglobin and capillaries Hydrolyse ATP faster to contract more quickly Describe the characteristics of fast glycolytic twitch muscle fibres - CORRECT ANSWER Few mitochondria and capillaries Less myoglobin Large concentration of glycogen for anaerobic respiration What structures make up the nervous system? - CORRECT ANSWER Brain, spinal cord, nerves What is the scientific name for nerve cells? - CORRECT ANSWER Neurones Name the organelles of the neurone - CORRECT ANSWER Dendrite, cytoplasm, nucleus, soma, axon, myelin sheath, axon terminal, Schwann cell, node of Ranvier What parts of the nervous system make up the central nervous system? - CORRECT ANSWER Brain and spinal cord Which parts of the nervous system make up the peripheral nervous system? - CORRECT ANSWER Neurones Define the term 'synapse' - CORRECT ANSWER The small gap in between the axon terminal of one neurone and the dendrite of the next neurone Define the term 'action potential - CORRECT ANSWER The impulse passed along the axon Define the term 'resting potential - CORRECT ANSWER When the neurone is not transmitting an action potential. During this time the K+/Na+ pump is at work What are the 5 sensory receptors? - CORRECT ANSWER Touch, taste, smell, hearing, sight Describe the myelin sheath - CORRECT ANSWER A thick insulating layer around the axon Describe the dendrites - CORRECT ANSWER Highly branched fibres that conduct impulses Describe the axon - CORRECT ANSWER A long single fibre that carries nerve impulses Describe the Schwann Cell - CORRECT ANSWER A cell wrapped around the axon, forming the myelin sheath Describe the nodes of Ranvier - CORRECT ANSWER Gap in the myelin sheath where the axon is exposed Describe the differences between myelinated and non-myelinated cells - CORRECT ANSWER Myelinated cells are longer and can transmit impulses faster down the axon Why do myelinated neurones transmit action potentials quicker than non-myelinated - CORRECT ANSWER Insulated by myelin sheath Impulse 'jumps' from node to node (where the sodium gates are located) What is the potential difference across the axon membrane during resting potential? - CORRECT ANSWER -70mV What is the potential difference across the axon membrane during an action potential? - CORRECT ANSWER +35mV What charge does the inside of an neurone have when it is polarised? - CORRECT ANSWER Negative What charge does the inside of a neurone have when it is depolarised? - CORRECT ANSWER Positive During resting potential which ions are being moved into the cell and which ions are moved out of the cell? - CORRECT ANSWER Na+ removed from the cell, K+ added to the cell How many sodium/potassium ions are removed/added in via the Na+/K+ pump? - CORRECT ANSWER 3 Na+ ions removed, 2 K+ ions added When does the first voltage gate open? - CORRECT ANSWER When the axon is stimulated and this reaches above potential threshold Which voltage gates are open during resting potential? - CORRECT ANSWER None Which voltage gates are open during depolarisation? - CORRECT ANSWER Sodium voltage gates Which voltage gates are open during repolarisation? - CORRECT ANSWER Potassium voltage gates What occurs during depolarisation? - CORRECT ANSWER Na+ voltage gates open, Na+ floods in causing the potential difference to increase What occurs during repolarisation? - CORRECT ANSWER K+ voltage gates open, K+ floods out of the cell causing potential different to decrease What is meant by the term hyper-polarisation? - CORRECT ANSWER When the cell becomes too negative due to the loss of too many K+ What is meant by the term saltatory conduction? - CORRECT ANSWER When the impulse jumps between nodes increasing the speed at which the impulse travels down the axon Which voltage gate opens in the synaptic bulb as the action potential reaches it? - CORRECT ANSWER Calcium voltage gate What happens in the synaptic bulb as the calcium voltage gates open? - CORRECT ANSWER Ca2+ floods in, stimulating the vesicles to move to the membrane of the presynaptic bulb Which chemical is held in the vesicles? - CORRECT ANSWER Acetylcholine (a neurotransmitter) Define the term exocytosis - CORRECT ANSWER The release of the acetylcholine into the synaptic cleft. This then diffuses across the synapse. What causes the sodium channel to open on the postsynaptic neurone? - CORRECT ANSWER Acetylcholine bonding to receptors on sodium voltage gate. What happens to the acetylcholine after the sodium channel opens? - CORRECT ANSWER Broken down by acetylcholinesterase into ethanoic acid and choline. This is then reabsorbed by the presynaptic neurone and reformed into acetylcholine. What does EEG stand for? - CORRECT ANSWER Electroenchephalogram Define Oscillation - CORRECT ANSWER A regularly repeating motion about central value What is frequency? - CORRECT ANSWER The number of whole cycles occurring in one second What is the formula for frequency? - CORRECT ANSWER f = 1/T What is the Period of a wave? - CORRECT ANSWER The time taken for one whole cycle of an oscillation. What is Displacement of a wave? - CORRECT ANSWER How far the quantity that is in oscillation has moved from its mean (rest) value. What is the amplitude of a wave? - CORRECT ANSWER The maximum value of displacement in the oscillation cycle. Always measured from the mean position. Explain a wave - CORRECT ANSWER Waves transfer energy from one point to another without causing any net movement of material. What is wavelength? - CORRECT ANSWER The distance along the wave in its direction of travel (propagation) between consecutive points where the oscillations are in phase. What is the wave equation? - CORRECT ANSWER v = fλ What is Phase Difference? - CORRECT ANSWER The difference between two waves of the same frequency and wavelength where 360degrees represents a single whole cycle. How do particles behave in a longitudinal wave? - CORRECT ANSWER The particles are displaced in the same direction that the wave travels. How do the particles behave in a transverse wave? - CORRECT ANSWER In a transverse wave the displacement is at right angles to the direction of the wave travel. What are the two parts of a longitudinal wave. - CORRECT ANSWER Compression (squashed) Rarefaction (spread out) What are the 2 types of wave? - CORRECT ANSWER Longitudinal And Transverse. What is diffraction? - CORRECT ANSWER Diffraction is the tendency of a wave to spread out in all directions. What is transmission of a wave? - CORRECT ANSWER The wave energy passing through an object and mostly continuing forward in the original direction. What is reflection? - CORRECT ANSWER Wave energy that bounces of a surface and has its direction of travel altered by 180degrees What is an interference pattern? - CORRECT ANSWER A stationary pattern that can result from the superposition of waves travelling in different directions provided they are coherent. What is coherence of a wave? - CORRECT ANSWER Superposition that causes a visible interference pattern. Must share the same wavelength and constant phase difference What is superposition? - CORRECT ANSWER The adding together of wave displacements that occurs when waves from 2 or more separate sources overlap. They add together. What is path difference. - CORRECT ANSWER The difference in length between 2 straight rays. What happens to a light at a point of constructive interference? - CORRECT ANSWER The light becomes more intense. Brighter. What happens to light at a destructive boundary? - CORRECT ANSWER The waves are cancelled out so there is a dark spot. What is the formula for Young's slit experiment? - CORRECT ANSWER nλ = d sin θ What is a photon? - CORRECT ANSWER A Quantum of electromagnetic radiation. Mass and charge = 0 What is quantum? - CORRECT ANSWER The smallest unit that can independently exist. What is Quantum Theory? - CORRECT ANSWER A combination of ideas from wave and particle mechanics How is the relationship between frequency and the energy of a photon expressed? - CORRECT ANSWER E = hf What is Planck's Constant? - CORRECT ANSWER -6.626 x 10^-34 Define the energy level of an electron. - CORRECT ANSWER One of the fixed, allowed, values of energy for an electron that is bound to an atom. What is ground state? - CORRECT ANSWER The lowest energy state possible for a given bound particle. What is de-excitation of an electron? - CORRECT ANSWER The return of an electron from an outer shell to the ground state. What is c, the speed of light? - CORRECT ANSWER 3 x 10^8 m/s What is ΔE? - CORRECT ANSWER The energy difference between the levels. The lost energy must equal the energy of the emitted photon. What is a Stationary or Standing Wave? - CORRECT ANSWER Wave motions that store energy rather than transferring energy to other locations. What is a Node? - CORRECT ANSWER Points along a stationary wave where displacement amplitude is at a minimum. (ideally zero) What is an Antinode? - CORRECT ANSWER Points of maximum amplitude that occur halfway between each pair of nodes. What is Resonance? - CORRECT ANSWER The storing of energy in an oscillation or stationary wave, the energy coming from an external source of appropriately matched frequency What is forcing frequency? - CORRECT ANSWER The frequency of wave energy from an external source that is coupled to a resonator. What is Natural Frequency? - CORRECT ANSWER A resonator has a series of natural frequencies. Each of which corresponds to an exact number of half wavelengths within its boundaries. What is this formula? - CORRECT ANSWER Wave speed formula for a standing wave What is Refractive Index? - CORRECT ANSWER The ratio of speed of light in a vacuum to its speed of light in the medium. What is the formula for refractive index? - CORRECT ANSWER The ratio of the speed of light, c, to the speed of light in the medium, v, is called the refractive index, n, of the medium. n=c/v What is the Normal Line of light? - CORRECT ANSWER The light at right angles to the surface of a transparent medium. What is incidence of a line? - CORRECT ANSWER The direction of the incoming ray. What is refraction? - CORRECT ANSWER Means bending of direction of travel, so it describes the direction of an outgoing ray after bending. What is the mathematical formula for refraction? - CORRECT ANSWER n = c/v = sin i / sin r What is Internal Reflection? - CORRECT ANSWER When a wave that is already in an optically dense medium hits the boundary with a less dense medium the energy is reflected back into the denser material What is the critical angle? - CORRECT ANSWER The angle of incidence where the angle of refraction is 90degrees What is Total Internal Reflection? - CORRECT ANSWER All the wave energy is internally reflected, none is lost as a refracted ray. What is the equation for total internal reflection? - CORRECT ANSWER 1/n = v/c = sin C => Sin C = 1/n What is an optical fibre? - CORRECT ANSWER Very long, thin cylinders of glass or plastic where light is totally internally reflected. What is the benefit of optical fibre? - CORRECT ANSWER They are a much more efficient way of transmitting data. What is a medical use of optical fibres? - CORRECT ANSWER Endoscopes. What is an analogue signal? - CORRECT ANSWER A signal with strength proportional to the quantity it is representing. What is a digital signal? - CORRECT ANSWER Conveys in binary code a number that represents the size of measured quantity. How fast do electromagnetic waves travel in a vacuum? - CORRECT ANSWER All electromagnetic waves travel at the same speed in a vacuum. 3 x 10^8 What is the formula for the area of a sphere? - CORRECT ANSWER 4π r^2 What is the formula for the intensity of a wave front? - CORRECT ANSWER l = k / r^2 Why does the intensity of a wave front change? - CORRECT ANSWER Because the wave is spreading out in all directions. Name the parts of a wave. - CORRECT ANSWER Peak, Trough, Wavelength, amplitude. Name the parts of the electromagnetic spectrum. - CORRECT ANSWER Radio waves, Microwaves, Infrared, Visible light, Ultraviolet light, X-Rays, Gamma Rays [Show More]
Last updated: 10 months ago
Preview 1 out of 26 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 08, 2023
Number of pages
26
Written in
Additional information
This document has been written for:
Uploaded
Aug 08, 2023
Downloads
0
Views
56