SCIENCE 101 > GIZMOS > GIZMOS Student Exploration_Calorimetry Lab: Vocabulary: calorie, calorimeter, joule, specific heat c (All)
GIZMOS Student Exploration_Calorimetry Lab: Vocabulary: calorie, calorimeter, joule, specific heat capacity. Score 100%
Document Content and Description Below
Student Exploration_Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “h... eat,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter does? 2. Where have you heard the word calorie before? What do you think a calorie is? Gizmo Warm-up A calorimeter is an insulated container filled with a liquid, usually water. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat capacity. You will use the Calorimetry Lab Gizmo™ to determine the specific heat capacities of various substances. 1. On the SIMULATION pane, select Copper. Use the slider to set its Mass to 200 g. Set the Water mass to 200 g. Check that the Water temp is set to 30.0 °C and the copper’s Temp is 90 °C. Select the GRAPH tab, and click Play ( ). A. What was the Final temperature of the copper and the water? B. How much did the temperature of the copper change? C. How much did the temperature of the water change? 2. Specific heat capacity can be described as a substance’s resistance to temperature changes. Which substance has a greater specific heat capacity, copper or water? Explain. Activity A: Heat transfer Get the Gizmo ready: ● Click Reset ( ). Question: What factors determine how heat energy transfers between objects? 1. Predict: In the Gizmo warm-up, you saw how 200 g of 90 °C copper transfers heat to 200 g of 30.0 °C water. A. How do you think increasing the water’s mass would affect the final temperature? B. How do you think decreasing the copper’s mass would affect the final temperature? C. How do you think increasing or decreasing the copper’s initial temperature would affect the final temperature? 2. Collect data: Use the Gizmo to determine the final temperature for each set-up listed below. Record your results in the tables. In the first table, you experiment with changing the water’s mass. In the second table, you change the copper’s mass. In the third table, you change the initial temperature of the copper. The first row of each table has been completed for you. Copper Water Final Temp. (°C) Initial Temp. (°C) Mass (g) Initial Temp. (°C) Mass (g) 3. 90 °C 200 g 30.0 °C 34.96 °C 90 °C 200 g 30.0 °C 30.54C 4. 90 °C 30.0 °C 200 g 34.96 °C 90 °C 30.0 °C 200 g 30.54C 5. 200 g 30.0 °C 200 g 34.96 °C 200 g 30.0 °C 200 g 35.79C 200 g 30.0 °C 200 g 31.65C 3. Analyze: For each factor listed in the chart below, explain how the final temperature was changed and why you think that change occurred. A. What was the effect of increasing the water’s mass? B. What was the effect of decreasing the copper’s mass? C. What was the effect of changing the initial temperature of the copper? 4. Draw conclusions: The amount that the water’s temperature increases depends on the mass of the water and the amount of heat energy in the copper. A. How does changing the initial mass of the copper affect how much heat energy it has? B. How does changing the initial temperature of the copper affect how much heat energy it has? 5. Apply: Many gyms and health clubs have steam saunas, which are small steam-filled rooms. Traditionally, steam saunas have a container of heated rocks. A small ladle of water is poured on the rocks in order to make the steam. A. Use what you have learned so far about heat transfer to explain how hot rocks can be used to make steam? B. Why do you think only a small ladle-full of water is poured on the rocks at one time? Activity B: Specific heat Get the Gizmo ready: ● Click Reset. ● Deselect Copper, and select Granite. Question: How can you compare the specific heat capacities of various substances? 1. Explain: How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the Gizmo? 2. Predict: Which substance do you think will have the highest specific heat capacity? Why? 3. Experiment: Use the Gizmo to determine the final temperature for each set-up listed below. Record your results in the table. The first row has been completed for you. Substance Substance initial temp. (°C) Substance mass Water initial temp. (°C) Water mass Final temp. (°C) Copper 90 °C 200 g 30.0 °C 200 g 34.96 °C Granite 90 °C 200 g 30.0 °C 200 g 39.59C Lead 90 °C 200 g 30.0 °C 200 g 40.83C 4. Analyze: Of the three substances, which caused the largest temperature change in the water? What does this indicate about its relative specific heat capacity? 5. Interpret: Remember that specific heat capacity is a measure of a substance’s resistance to temperature change. The more resistant a substance is to temperature change, the higher is its specific heat capacity. Rank the three substances in order of their specific heat capacities, from highest to lowest. 6. Predict: How do you think the specific heat capacity of ice will compare to that of copper, granite, and lead? 7. Experiment: Deselect Lead, and select Ice. Use the default values for Temp (-30 °C) and Mass (50 g). Set the Water temp to 60 °C and the [Show More]
Last updated: 1 year ago
Preview 1 out of 10 pages

Reviews( 0 )
Recommended For You
Chemistry> GIZMOS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity (All)
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “...
By Professor Lynne , Uploaded: Oct 06, 2021
$9.5
Physics> GIZMOS > Gizmos Student Exploration| Calorimetry Lab| Calorie, calorimeter, joule, specific heat capacity. Download for Grade A+ (All)
Gizmos Student Exploration| Calorimetry Lab| Calorie, calorimeter, joule, specific heat capacity. Download for Grade A+
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor m...
By Quiz Ace , Uploaded: May 13, 2021
$10
Physics> GIZMOS > Gizmos Student Exploration| Calorimetry Lab| Calorie, calorimeter, joule, specific heat capacity. Exam Graded A+ (All)
Gizmos Student Exploration| Calorimetry Lab| Calorie, calorimeter, joule, specific heat capacity. Exam Graded A+
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor m...
By Quiz Merchant , Uploaded: May 08, 2021
$10
SCIENCE 101> GIZMOS > GIZMOS.Student Exploration: Calorimetry Lab SE<calorie, calorimeter, joule, specific heat capacity> CORRECTLY DONE.100% SCORE (All)
.png)
GIZMOS.Student Exploration: Calorimetry Lab SE<calorie, calorimeter, joule, specific heat capacity> CORRECTLY DONE.100% SCORE
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “heat...
By Expert Tutor , Uploaded: Apr 21, 2021
$10
Biology> GIZMOS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. Graded. 100% (All)
.png)
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. Graded. 100%
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. Graded. 100%
By quizprof , Uploaded: Apr 13, 2021
$13
Physics> GIZMOS > Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. GRADED A+ (All)
.png)
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. GRADED A+
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity. GRADED A+
By quizprof , Uploaded: Mar 10, 2021
$13
Chemistry> GIZMOS > Student Exploration Density Laboratory GIZMOs Answer Key [TOP RATED] (All)

Student Exploration Density Laboratory GIZMOs Answer Key [TOP RATED]
Student Exploration: Density Laboratory Vocabulary: buoyancy, density, graduated cylinder, mass, matter, scale, volume
By jakesuli , Uploaded: Jul 19, 2021
$9
Botany> GIZMOS > Student Exploration, Seed Germination. Gizmos (All)
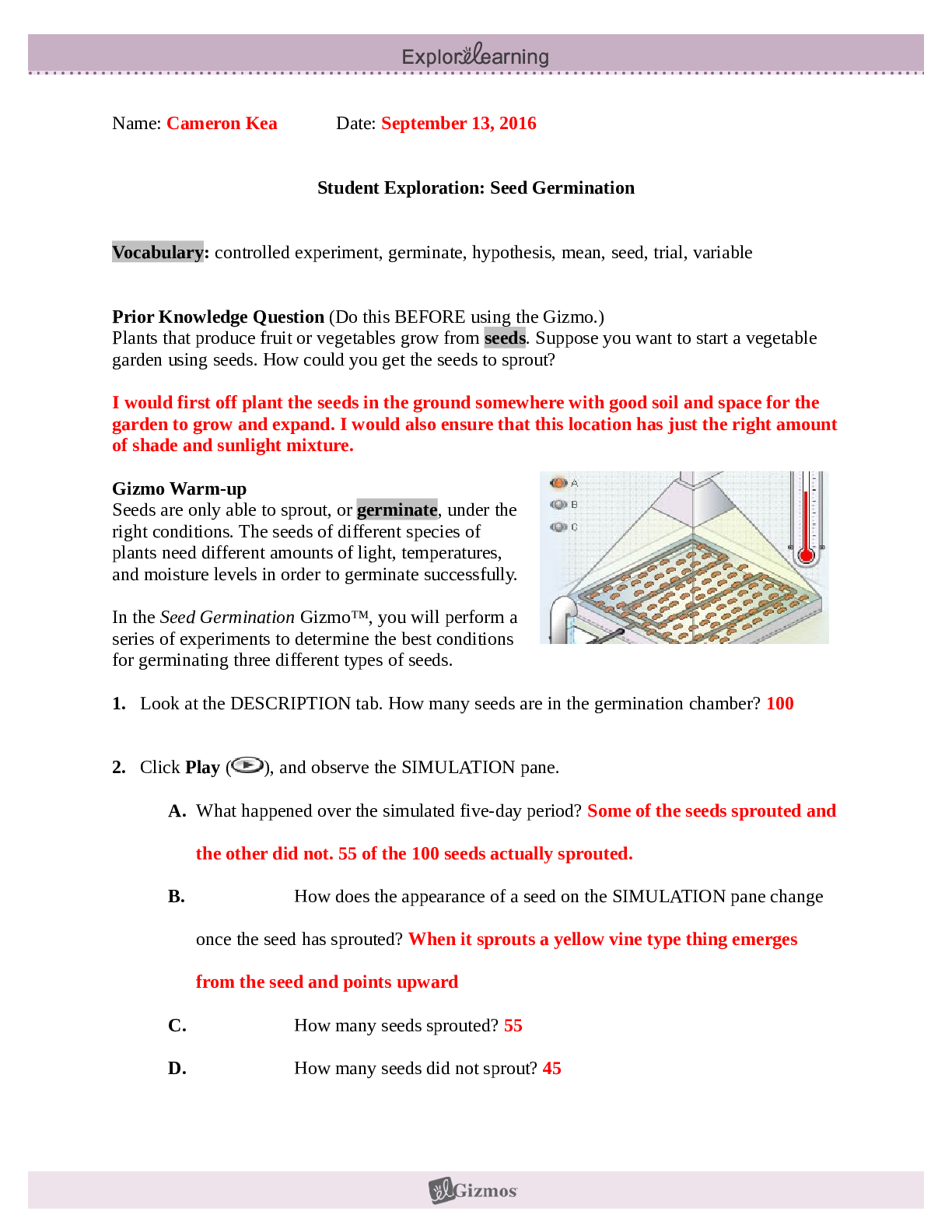
Student Exploration, Seed Germination. Gizmos
Prior Knowledge Question (Do this BEFORE using the Gizmo.) Plants that produce fruit or vegetables grow from seeds. Suppose you want to start a vegetable garden using seeds. How could you get the se...
By QuizGuider82 , Uploaded: Jul 18, 2021
$9
Biology> GIZMOS > Student Exploration, Mouse Genetics, Gizmos Student Exploration Sheet (One Trait) (All)
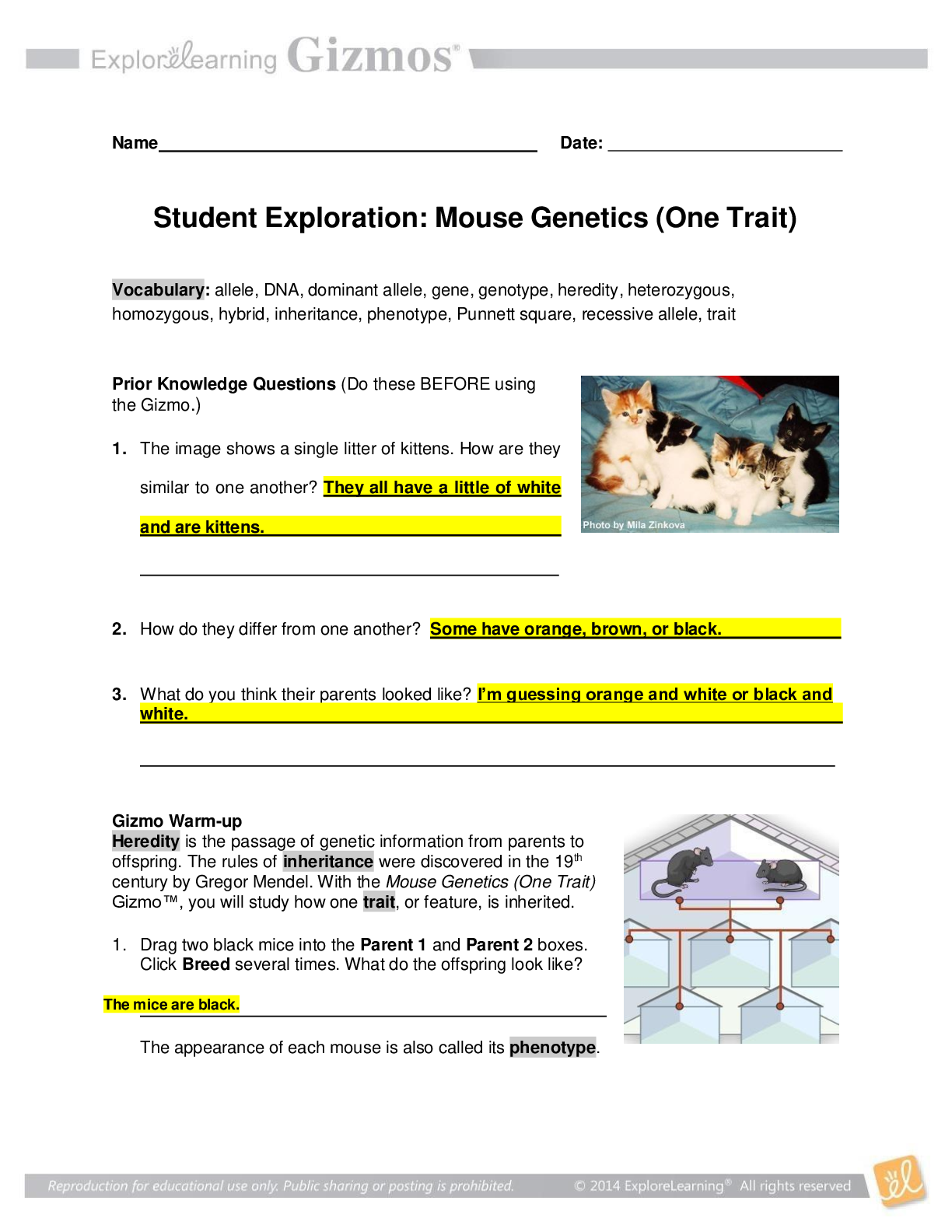
Student Exploration, Mouse Genetics, Gizmos Student Exploration Sheet (One Trait)
Student Exploration: Mouse Genetics (One Trait) Vocabulary: allele, DNA, dominant allele, gene, genotype, heredity, heterozygous, homozygous, hybrid, inheritance, phenotype, Punnett square, recessiv...
By Dr Medina Reed , Uploaded: Oct 10, 2021
$7
Chemistry> GIZMOS > Explore Learning. Student Exploration: Ionic Bonds. GIZMO. Questions and Answers > (All)
.png)
Explore Learning. Student Exploration: Ionic Bonds. GIZMO. Questions and Answers >
Name: Hannah Robb Student Exploration: Ionic Bonds Vocabulary: chemical family, electron affinity, ion, ionic bond, metal, nonmetal, octet rule, shell, valence electron, ♫heavy metal ♫...
By Kirsch , Uploaded: Dec 22, 2020
$11
Document information
Connected school, study & course
About the document
Uploaded On
Mar 21, 2023
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
Mar 21, 2023
Downloads
0
Views
66






