Sociology > QUESTIONS & ANSWERS > Social-Behavioral-Educational (SBE) Comprehensive, All unit tests (answered spring 2023) (All)
Social-Behavioral-Educational (SBE) Comprehensive, All unit tests (answered spring 2023)
Document Content and Description Below
Social-Behavioral-Educational (SBE) Comprehensive: Citi program (all quizzes) Answered all correctly_2023. Informed Consent - SBE (Answered) A therapist at a free university clinic treats elementa... ry school children with behavior problems who are referred by a social service agency. She is also a doctoral candidate who proposes using data she has and will collect about the children for a case-based research project. Which of the following statements about parental permission is correct? A general requirement for informed consent is that no informed consent may include any exculpatory language. Exculpatory language is that which waives or appears to waive any of the subject's legal rights or releases or appears to release those conducting the research from liability for negligence. Which of the following statements in a consent form is an example of exculpatory language? A criterion for waiving informed consent is that, when appropriate, subjects are provided additional pertinent information after the study. In which of the following studies would it NOT be appropriate to provide subjects with information about missing elements of consent: A waiver of the requirement for documentation of informed consent may be granted when: As part of the consent process, the federal regulations require researchers to: CITI Privacy and Confidentiality SBE (answered Quiz) A researcher leaves a research file in her car while she attends a concert and her car is stolen. The file contains charts of aggregated numerical data from a research study with human subjects, but no other documents. The consent form said that no identifying information would be retained, and the researcher adhered to that component. Which of the following statements best characterizes what occurred? Which of the following constitutes both a breach of confidentiality (the research data have been disclosed, counter to the agreement between researcher and subjects) and a violation of subjects' privacy (the right of the individuals to be protected against intrusion into their personal lives or affairs)? In a longitudinal study that will follow children from kindergarten through high school and will collect information about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable information? When a focus group deals with a potentially sensitive topic, which of the following statements about providing confidentiality to focus group participants is correct? Data are made anonymous by Assessing Risk - SBE (Quiz Answered) If disclosure of a subject's involvement in a specific research study can be potentially harmful to the subject, and the consent form is the only record linking the subject to the research, which of the following would be most helpful: The primary purpose of a Certificate of Confidentiality is to: Additional safeguards that may be included in a social and behavioral study may include: A researcher wants to do a web-based survey of college students to collect information about their sexual behavior and drug use. Direct identifiers will not be collected; however, IP addresses may be present in the data set. Risk of harm should be evaluated by: Identify the example of when situation and time are key to assessing risk of harm in a research study: Defining Research with Human Subjects - SBE CITI According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which of the following studies meets the definition of research with human subjects? Census data is an example of: According to the federal regulations, human subjects are living human beings about whom an investigator obtains data through interaction or intervention with the individual or: A professor at Big State University is writing a biography about Bill Gates and conducting oral histories with all of Bill Gates' friends, family members and business acquaintances. The researcher submits the research proposal to the institution's IRB. What action can he expect by the IRB? Conflicts of Interest in Human Subjects Research (ID 17464) What is the term for management controls that are built in to a research study (for example, independent data analysis)? When developing conflict of interest management plans, COI committees typically examine the study design to determine whether it includes inherent controls that mitigate the researcher's opportunity to bias the research. Inherent controls may include independent data analysis, randomization, blinding, or low subject enrollment percentage at a local site for a large multi-center trial. A researcher calls you stating that he plans to submit a proposal to the NIH for a human subjects research study. He wants to know at what point he and his study team must submit COI disclosures to comply with the PHS regulation. The NIH is a PHS agency. Therefore, this proposed research is subject to the PHS regulation regarding objectivity in research, which requires researchers to submit COI disclosures no later than the time a proposal is submitted to a PHS funding agency. The PHS regulations about financial conflict of interests require which party to disclose significant financial conflicts of interest? The PHS regulations about financial conflict of interests require the researcher to disclose significant financial conflicts of interest to the organization. The FDA's regulation governing disclosure of individual COI requires applicants submitting marketing applications for drugs, biologics, or devices to certify the absence of certain financial interests or disclose financial interests of researchers who conducted clinical studies covered by the regulation. An example of an individual financial COI is Conflicts of interest may arise in the peer review process (for example, bias causes a reviewer to respond positively to a manuscript because it involves research or methodology in which the reviewer has a personal interest). The peer review process can create conflicts of interest because the choice of who reviews a potentially publishable project may show: The COI management plan aims to: A COI management plan is a document that explains the procedures or extra steps to be taken to minimize the risk of bias. The procedures or protections put into place to minimize the risk of bias are often called controls. Management plans are typically tailored to the study and the researcher's financial interests. Management plans are not designed to eliminate COIs, nor reduce IRB regulatory review burden. Although management plans may be used for single site or multi-center research, their aim is to provide controls not just address disclosure of COIs. An example of an institutional COI is: An institutional COI can arise when the financial interests of an organization or institutional official (acting within his or her authority on behalf of the organization) may affect or appear to affect the research conducted under the organization's auspices. This could include significant gifts received by the organization from the sponsor of human subjects research The FDA regulations governing disclosure of individual COIs require: An individual COI may arise when an individual has a personal or financial interest, which may affect or appear to affect the design, conduct, or reporting of the research. A researcher's membership on an advisory board with an organization sponsoring research can create a COI because: The FDA's regulation governing disclosure of individual COIs requires applicants submitting marketing applications for drugs, biologics, or devices to certify the absence of certain financial interests or to disclose financial interests of researchers who conducted clinical studies covered by the regulation. The regulation specifies that the FDA may refuse to file any marketing application that does not contain a disclosure of researchers' financial interests or a certification that the applicant acted with due diligence to obtain researchers' disclosures, but was unable to do so. A researcher's membership on an advisory board of an entity sponsoring research can create a conflict of interest because there may be a perception that the researcher has a motive to bias the research to create an outcome that is favorable for the sponsor. During an Institutional Review Board (IRB) meeting, any IRB member who may have a potential COI with a study under review should: IRB policies and procedures generally specify that members with conflicts of interest related to an agenda item must disclose their conflicts of interest, and may answer questions from the IRB about the item with which they have a conflict, but are prohibited from voting on that item. A researcher's membership on an advisory board with an organization sponsoring research can create a COI because: A researcher's membership on an advisory board of an entity sponsoring research can create a conflict of interest because there may be a perception that the researcher has a motive to bias the research to create an outcome that is favorable for the sponsor. Populations in Research Requiring Additional Considerations and/or Protections A subject participates in a drug study because treatment is available at no or reduced cost, and he could not otherwise afford it. This is an example of According to the authors, there are four common abuses that historically are described as giving rise to vulnerability . Which response below contains the correct four? In considering NBAC's analytic approach, an otherwise competent person who is acutely ill might be considered at especially high risk of harm for: NBAC proposed a concept of vulnerability in research based on features of potential subjects or of their situation. Which of the following was NOT included as possibly leading to vulnerability? Identify the following groups that are protected in the federal regulations (45 CFR 46), specifically in Subparts B, C and D with additional protections: When an IRB is reviewing a research study and they are considering if a potential subject population is vulnerable, they should consider: Subjects with a serious illness may be at risk for exploitation since they may be desperate for a possible cure. This is an example of Which is true of inducements in research? Research Involving Workers/Employees SBE CITI QUIZ 2023. Vulnerable persons are those who are less able to protect themselves than other persons in a given situation. The Common Rule (45 CFR 46) has specific requirements for the following vulnerable populations, except: When workers are asked to participate in a research study, vulnerabilities related to the subject's employment may include: Researcher access to confidential records adds to the vulnerability of workers who participate in workplace studies. Inappropriate release of identifiable private information could adversely affect a worker's retention of a job, insurance or other employment related benefits. To avoid or minimize these risks, the study design must include adequate safeguards to protect the confidentiality of the information collected. A plan for the proper management of study data and records should clearly define: True or False: When a research project includes the collection of biological samples, all planned future uses of the samples, identifiers, and the data obtained from the samples, must be fully explained to the research subject. Social and Behavioral Research - Unanticipated Problems and Reporting Requirements in Social and Behavioral Research A researcher conducts a focus group to learn about attitudes towards hygiene and disease prevention. Two weeks after the focus group, the researcher learns one of the subjects had a heart attack at home and was hospitalized, but made a full recovery. Based on HHS regulations, should the researcher report this event to the IRB? Researchers must report potential unanticipated problems that involve risks to others directly to the: The procedures for reporting potential unanticipated problems involving risk to subjects or others to the IRB are: A researcher conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. The data are stored on a laptop computer without encryption, and the laptop computer is stolen from the researcher's car on the way home from work. This is an unanticipated problem that must be reported because the incident was (a) unexpected (in other words, the researchers did not anticipate the theft); (b) related to participation in the research; and (c) placed the subjects at a greater risk of psychological and social harm from the breach in confidentiality of the study data than was previously known or recognized. According to OHRP, this unanticipated problem must be reported to the IRB in which timeframe? A researcher conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. One of the subjects is in an automobile accident two weeks after participating in the research study. Is this an example of an unanticipated problem that requires reporting to the IRB? . According to OHRP, a problem is an "unanticipated problem" when it meets which of the following criteria: Unanticipated Problems and Reporting Requirements in Social and Behavioral Research- SBE (QUIZ) A researcher conducts a focus group to learn about attitudes towards hygiene and disease prevention. Two weeks after the focus group, the researcher learns one of the subjects had a heart attack at home and was hospitalized, but made a full recovery. Based on HHS regulations, should the researcher report this event to the IRB? Researchers must report potential unanticipated problems that involve risks to others directly to the: The procedures for reporting potential unanticipated problems involving risk to subjects or others to the IRB are: A researcher conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. The data are stored on a laptop computer without encryption, and the laptop computer is stolen from the researcher's car on the way home from work. This is an unanticipated problem that must be reported because the incident was (a) unexpected (in other words, the researchers did not anticipate the theft); (b) related to participation in the research; and (c) placed the subjects at a greater risk of psychological and social harm from the breach in confidentiality of the study data than was previously known or recognized. According to OHRP, this unanticipated problem must be reported to the IRB in which timeframe? A researcher conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. One of the subjects is in an automobile accident two weeks after participating in the research study. Is this an example of an unanticipated problem that requires reporting to the IRB? Internet-Based research SBE CITI Which of the following methods could be considered a "best practice" in terms of informing respondents how their answers to an on-line survey about personal information will be protected? Which of the following examples of using the Internet to conduct research meets the federal definition of research with human subjects? Consent to participate in research is an ongoing process. Which of the following strategies would help ensure that participation in a survey about a sensitive personal topic remains voluntary throughout a study? To minimize potential risks of harm, a researcher conducting an on-line survey can: - The Internet can be used as a research tool or as the object of a study. Which of the following examples best describes an investigator using the Internet as a research tool? Internet-Based Research - SBE (ID 510) Which of the following examples of using the internet to conduct research meets the federal definition of research with human subjects? The federal regulations define both a human subject and research separately, but both definitions must be met to quality as human subjects research. Thus, research with human subjects is defined as a systematic investigation (intent to contribute to generalizable knowledge) involving living individuals about whom a researcher conducting research obtains information through intervention or interaction with the individual, and uses, studies, or analyzes the information or obtains, uses, studies, analyzes, or generates identifiable private information (Protection of Human Subjects 2018). Conducting an on-line focus group with live human beings to research familial support systems would therefore meet the definition of research with human subjects. Analyzing the number of visits to a website provides information about the website itself not about the people who visit it and does not meet the federal definition of research with human subjects. Neither does gathering information about deceased individuals. Using data that are publically available and de-identified are no longer private or personally identifiable, and would also not meet the federal definition of research with human subjects. Consent to participate in research is an ongoing process. Which of the following strategies would help ensure that participation in a survey about a sensitive personal topic remains voluntary throughout a study? Voluntary participation in research includes the right to withdraw from a study at any time and the right to choose not to answer questions. On-line surveys that force subjects to answer one question before going to the next violate the requirement that all participation in research be voluntary. Providing contact information for the researcher, privacy policies, and description of the survey are important, but they do not address the requirement. Which of the following on-line research strategies raises the most concerns regarding the ethical principle of respecting the autonomy of research subjects and the corresponding federal regulations requiring informed consent? - The study in which the researcher pretends to be a cancer survivor involves deception and research without any informed consent process. Therefore, the study raises concerns about the ethical principle of respect for persons. Any compromise of the principle should be justified by any scientific benefit to be derived from the study. When a support group or a blog is open to any and all readers, it can be argued that the communications posted are not intended to be private and can therefore be observed and recorded without informed consent. The researcher studying interracial adoptees used the internet in a manner comparable to posting a flyer on a bulletin board. - Which of the following methods could be considered a "best practice" in terms of informing respondents how their answers to an on-line survey about personal information will be protected? Although there are no guaranteed methods to assure absolute confidentiality of research data collected on-line, some internet-based research experts have identified "best practices" for describing commonly accepted confidentiality protections, such as including explanations about how data are transmitted from the subject to the investigator, how the investigator will maintain and secure the data. Consent processes should also include a discussion to emphasize that there is no way to guarantee absolute confidentiality if data are of a personal or sensitive nature. The internet can be used as a research tool or as the object of a study. Which of the following examples best describes an investigator using the internet as a research tool? Investigators use the internet as a research tool when they actively engage, or interact, with their participants on-line. This can include distributing an instrument via email or hosting a web-based survey on an on-line survey provider, recruiting subjects from on-line panels, conducting interviews on-line, facilitating focus groups in private chat rooms, and posting on-line experiments or interventions on web-based service providers. Researchers endeavoring to conduct an on-line study should consider that there are some potential risks of harm to subjects unique to internet-based research. One of these risks is: Information posted on-line could, conceivably, be accessible to anyone with an internet connection. However, people may post their private identifiable information on-line without the intention of this information being public. Private identifiable information that can be accessible to anyone can create the potential for several different risks of harm to subjects who may not be aware that their information is public. Assuming a pseudonymous on-line identity does not pose any risks of harm to subjects. Though it is true that researchers can recruit, consent and debrief subjects on-line with little to no-interaction, this in of itself, would also not pose any risks of harm to subjects. Similarly, waiving the documentation of consent does not pose any potential risks of harm to subjects, and is not unique to internet-based research. To minimize potential risks of harm, a researcher conducting an on-line survey can: - In most internet-based research, the primary risk of harm is a breach of confidentiality. A simple way to help maintain the confidentiality of a subjects' identity is not to collect direct or indirect identifiers. Suggesting to subjects that they should print a copy of the informed consent form does not protect them from an inadvertent breach of identifiable responses. Similarly, complying with the Terms of Service does not protect against a breach. Specifying that all subjects be of legal adult age does not ensure compliance. - International Research - SBE (ID 509) Which of the following is the least important activity when protecting human subjects in international research? It is essential that researchers have sufficient knowledge of the local research context to be able to design and carry out research in a way that protects the rights and welfare of the subjects. This includes knowledge about unique risks subjects might face given the local socio-economic conditions. Community consultation is particularly important when research has the potential to affect the community as a whole and when there are gatekeepers for the community (for example, a tribal council). - A professor at Big State University proposes to study attitudes about obesity in Chile by giving subjects in Chile surveys to complete. Which is a question that the Big State University IRB should ask the researcher in order to determine if this study should be reviewed by a local Chilean IRB or ethics committee, as well as the Big State University IRB? There are several factors that determine what kinds of review are needed for international research, including if the researcher have collaborators at the research site abroad. If international research is carried out without the involvement of foreign collaborators the research may be reviewed by the researcher's home institution. The need for local review will be determined by policies and regulations rather than by U.S. law. When U.S. researchers collaborate with researchers at foreign institutions, determining the appropriate review type and method depends on whether the collaborating institution is engaged in the research. The other responses are incorrect because they do not help the IRB determine where the research should be reviewed. - A researcher proposes to conduct a study at a foreign site. The research has been determined to be exempt from the federal regulations by institutional policy. According to federal regulations, is review required at the foreign site? What procedures must be described in an agreement called an "assurance of compliance" with a U.S. federal agency? A researcher proposes a study and wants to recruit subjects from health care clinics in Jamaica. The survey will be conducted by the U.S. researchers at the clinic. The nurses at the clinic will inform prospective subjects about the availability of the research, but will not consent the subjects nor perform any research procedures (even screening procedures). Are the nurses engaged in the research according to federal regulations? - A great deal of research in the social and behavioral sciences may qualify for exemption, depending upon institutional policy. If a proposed study qualifies for exemption, federal regulations do not require review at the foreign site where it will be conducted. Federal regulations do not require exempt research to be reviewed by an institution's IRB or an institutional official - those decisions are left to institutional policy. If research involves collaboration with an institution that is "engaged" in research in the foreign country, the collaborating institution will need to have procedures in place that ensure that subjects will be protected in a manner commensurate with the Common Rule, including review by an independent committee comparable to an IRB. These procedures must be described in an agreement called an "assurance of compliance" with a U.S. federal agency. The written procedures for reporting unanticipated problems are institutional policy, the procedures outlining randomization should be covered in the research plan/protocol, and the procedures in place to comply with ethical principles is the Federalwide Assurance (FWA), not "assurance of compliance." - No, they are not engaged because they are only informing the subjects and not consenting or performing any research procedures, or receiving or sharing any private, identifiable information. An institution is not engaged if its only involvement is to provide space for the U.S. researchers to conduct their own research. - Which of the following activities constitutes engagement in research? - The age of majority in international research is determined by the If an institution obtains informed consent and conducts research interviews it is engaged in research. - The age of majority should be the recognized age of majority in the country or region where the research takes place. - What are some considerations for a U.S. researcher conducting a study in a non-U.S. setting when obtaining informed consent from subjects? – International Research- SBE quiz 2023. What procedures must be described in an agreement called an "assurance of compliance" with OHRP? What are some considerations for a U.S researcher conducting a study in a non-U.S. setting when obtaining informed consent from subjects? A professor at Big State University proposes to study attitudes about obesity in Chile by giving subjects in Chile surveys to complete. Which is a question that the Big State University IRB should ask the researcher in order to determine if this study should be reviewed by a local Chilean IRB or ethics committee as well as the Big State University IRB? A Researcher proposes to conduct a study at a foreign site that has been determined to be exempt from the federal regulations by institutional policy. According to federal regulations, is review required at the foreign site? The age of majority in international research is determined by the A researcher proposes a study and wants to recruit subjects from health care clinics in Jamaica. The survey will be conducted by the U.S. researchers at the clinic. The nurses at the clinic will inform prospective subjects about the availably of the research, but will not consent the subjects nor perform any research procedures (even screening procedures). Are the nurses engaged in the research according to federal regulations? - Research in Public Elementary and Secondary Schools - SBE A student's disciplinary status is part of his or her school record. In accordance with FERPA, schools must usually have written parental permission before they can release data from school records to researchers. The Protection of Pupil Rights amendment is concerned with the kinds of questions that may be included in surveys and interviews with minor school children. The No Child Left Behind Act is a specific amendment to PPRA that gives parents additional rights over the content of research materials. Subpart D of 45 CFR 46 is concerned with the rights of children as research subjects and does not regulate school systems. - FERPA, PPRA, and Subpart D of the federal regulations also govern research in the public schools. - If research in a private school is directly funded by the Department of Education, PPRA applies, regardless of the risk level of the research (more than minimal or no more than minimal). A private school that does not receive any federal funding is not subject to the provisions of FERPA or PPRA. - If research in a private school is directly funded by the Department of Education, then: - If the research is subject to Subpart D, which of the following research activities with children would qualify for an exemption under Category 2 (research that includes educational tests, surveys, interviews, observation)? In addition to the general provisions of the Common Rule (the federal regulations for protecting research subjects), the following regulations also govern research in the public schools: Parental notification, in lieu of active parental permission, is allowed when: - Parental permission must be secured or waived in accordance with criteria established by federal regulation. When a waiver has been approved, investigators may wish to, and IRBs may require, that parents be notified that the study will take place, giving them the opportunity to withdraw their children from the study. Parental notification can never be substituted for active parental permission if the criteria for a waiver have not been met. - PPRA gives parents some level of control over their child's participation in third-party survey research or exposure to instructional materials developed by researchers. FERPA gives parents certain rights with respect to their children's educational records. The ability to opt out of health education would be an institutional, local or state law, and is not covered in this module about research. The access to medical records would be covered under Health Insurance Portability and Accountability Act (HIPAA) and is not covered in this module as it does not relate to research in public schools. - PPRA gives parents some level of control over their child's: - Participation in third-party survey research or exposure to instructional materials developed by researchers Schools may disclose, without consent, directory information such as a student's name, address, telephone number, date and place of birth, honors and awards, and dates of attendance. However, schools must tell parents and eligible students that directory information is not protected, and they must allow parents and eligible students a reasonable amount of time to request that the school not disclose directory information about them. FERPA allows schools to disclose identifiable records without permission to certain parties, including organizations conducting research initiated by a school district or a state department of public instruction. - Subpart D, Additional HHS Protections for Children, specifically prohibits the use of exemption for research involving survey procedures, interview procedures, or participant observation when the researcher participates in the activities being observed. - The purpose of FERPA is to give parents certain rights with regard to the release of their children's educational records. School personnel such as teachers, counselors, and principals may access student records for legitimate school functions. Generally, schools must have written permission from a parent before releasing any identifiable information from a student's record. - The purpose of the Family Educational Rights and Privacy Act (FERPA) is to: Which federal regulation or law governs how researchers can obtain data about subjects' disciplinary status in school from academic records? Which of the following types of information may schools disclose without consent from the parent or student to a researcher at a local university CITI Training: Research in Public Elementary and Secondary Schools If research in a private school is directly funded by the Department of Education, then: The purpose of the Family Educational Rights and Privacy Act (FERPA) is to: Which of the following types of information may schools disclose without consent from the parent or student to a researcher at a local university? Parental notification, in lieu of active parental permission, is allowed when: PPRA gives parents some level of control over their child's: The purpose of the Family Educational Rights and Privacy Act (FERPA) is to: (correct)- In addition to the general provisions of the Common Rule (the federal regulations for protecting research subjects), the following regulations also govern research in the public schools: Which federal regulation or law governs how researchers can obtain data about subjects' disciplinary status in school from academic records? PPRA PPRA also concerns marketing surveys and other areas of student privacy, parental access to information, and the administration of certain physical examinations to minors. The rights under PPRA transfer from the parents to a student who is 18 years old or an emancipated minor under state law. Parental notification, in lieu of active parental permission, is allowed when: - The researcher anticipates a low response rate. - An IRB has approved a waiver of the requirement for parental permission. - The researcher has conducted a similar study at another institution. - The superintendent of schools and the principals have approved the study. Research with Children – SBE The provisions of Subpart D, of the HHS regulations, Additional Protections for Children Involved as Subjects in Research apply to: A researcher wants to observe preschoolers at a local public playground to evaluate levels of cooperation. The researcher will not interact with the children or record information in such a manner that the identity of the subjects can be readily ascertained. Which of the following statements is true? Which of the following statements most accurately describes the requirement for the documentation of minors' assent to participate in research According to federal regulations, "children" are defined as: According to Subpart D, research with children may be eligible for exemption under Category 2 when: - Research with Children - SBE Quiz (Answered) The provisions of Subpart D, of the HHS regulations, Additional Protections for Children Involved as Subjects in Research apply to: The provisions of Subpart D must be applied to all research funded by the HHS (which includes NIH). However, not all federal agencies that have adopted the Common Rule have also adopted Subpart D. In addition to HHS, only the U.S. Food and Drug Administration and the Department of Education have adopted it. Institutions may elect to apply the subpart to all research, regardless of the source of funding. - A study that involves interviews of adults is eligible for expedited review. The researcher wants to add an adolescent population (aged 12 to 17) to the study and has designed a parental permission and assent process. No additional changes are planned. Which of the following statements about review of the revised protocol is accurate? - Research involving children may be expedited if the level of risk is no more than minimal and if the research falls into a category of research identified as eligible for expedited review. Therefore, unless the nature of the topic would raise the level to more than minimal risk to the adolescent subjects, the study previously approved for adults through expedited review procedures would also be eligible for expedited review. It is not necessary for adolescents to have obtained some adult rights through emancipation procedures, nor must the reading level of the subjects be predetermined in order for a study involving minors to be eligible for expedited review. Consent forms, including the readability of the information, must always be tailored to the particular subject population of a study. - A researcher wants to observe preschoolers at a local public playground to evaluate levels of cooperation. The researcher will not interact with the children or record information in such a manner that the identity of the subjects can be readily ascertained. Which of the following statements is true? The exemption categories that may be used with children include observations of children in public settings, as long as the researcher does not participate in the activities being observed or record information in such a manner that the identity of the subjects can be readily ascertained. Research does not require full committee review when it involves children, unless it does not fall into an exempt or expedited category. - The specific Department of Health and Human Services (HHS) regulations that apply to research with children are known as: The provisions of Subpart D, of the HHS regulations, Additional Protections for Children Involved as Subjects in Research are the specific Department of Health and Human Services (HHS) regulations that apply to research with children. Subpart B specifies Additional Protections for Pregnant Women, Human Fetuses and Neonates Involved in Research, Subpart C specifies Additional Protections Pertaining to Biomedical and Behavioral Research Involving Prisoners as Subjects and Subpart A is the Basic HHS Policy for Protection of Human Research Subjects (generally referred to as the Common Rule). - According to federal regulations, "children" are defined as: According to the federal regulations, children are persons who have not yet attained the legal age of consent under the applicable laws in the jurisdiction in which the research will be conducted. Generally, though not always, the age of consent is the age at which minors reach the age of majority and are considered adults. In the United States, state law dictates the age of majority. In most states, the age of majority is 18. This means that a 17-year-old may be considered a child when applying the federal regulations for protecting research subjects. - According to Subpart D, research with children may be eligible for exemption under Category 2 when: Subpart D restricts the use of exemptions when children are research subjects. Research that involves interviews, surveys, or participant observation when the researcher interacts with the children is not eligible for exemption under Category 2. Which of the following statements most accurately describes the requirement for the documentation of minors' assent to participate in research? The federal regulations do not require that child assent be documented. Therefore it is not necessary to request a waiver of a requirement to document assent. An IRB will determine whether and how documentation is required for a particular study. The document does not require parental input or parental review. – Research with Prisoners - SBE (ID 506) Quiz Which of the following statements about prison research is true? Because studies of the effects of processes of incarceration are allowed under the regulations, researchers may study the effects of privilege upgrades awarded by the prison. The regulations do not prohibit payment of prisoners for their participation. However, the amount of compensation cannot constitute undue influence to participate. Many correctional institutions may prohibit payment. The decision to participate in research may not be considered during parole hearings, as this practice would clearly constitute undue influence to participate. The regulations require that IRBs determine that risks involved in research with prisoners are commensurate with risks that would be accepted by non-prison volunteers. A graduate student wants to examine the effect of print media versus televised media on individuals' position on several social issues. The superintendent of a local work release facility, a family friend, will allow the graduate student access to the prison population to help her quickly accrue subjects. The student's IRB should: Factors such as the level of risk and proposed facility are irrelevant if the prison population is simply a population of convenience as it is in this case. Research taking place in prisons must be material to the lives of the prisoners. Therefore, according to the regulations, an IRB could not approve this protocol. A researcher's study uses a dataset of prisoner demographic characteristics. This dataset includes criminal history data that predates incarceration and includes data on disciplinary behavior while in prison. There is no interaction with prisoners. The researcher claims, and the IRB chair agrees, that the study is exempt from IRB review. This decision: The other answers are incorrect because they assume that an IRB can use an exempt status for prisoner research. The study could qualify for expedited review, but OHRP strongly discourages the use of expedited review procedures as an acknowledgement of the vulnerability of prisoners as a class. A researcher is examining the quality of life for prisoners who are HIV-positive using surveys followed by interview. The IRB must ensure that: One of your subjects is half way through a study of an investigational antidepressant that is injected weekly. The drug requires a taper-down regimen, that is, it should not be stopped abruptly. You learn that the subject will be admitted to prison next week prior to the next scheduled injection. What is the appropriate response for the researcher? - By participating in the research, it is possible that subjects will become publicly identified as HIV-positive. This breach of confidentiality could lead to negative consequences for the prisoner. One method for the investigator to preserve this confidentiality is to interview a larger sample of offenders, some who are HIV-positive and some who are not. While the survey should be validated and reliable, it does not have to be standardized. Because the research is behavioral only, there is no need for a medical doctor. The prison's HIV testing procedures are not part of the study. - Although the specific details are not known, the researcher should take an active role in ensuring that the subject's health is protected. The other answers are not sufficient in this case. A sociologist wants to study a culture that occurs in some women's prisons: "state families," in which individual prisoners take on certain roles within a group of like-minded prisoners. There is previous evidence that younger prisoners will use older inmates who play the roles of grandparents as a resource before they will turn to staff for help and advice. The lieutenant in charge of a dorm of long-term prisoners offers to gather volunteers to speak to the researcher and also offers to vouch for the integrity of the researcher. The use of this staff is: Using staff or prisoners to help select subjects leads to undue influence and coercion. This methodology is not "snowball sampling." There is no waiver for this type of recruitment. Which example of research with prisoners would be allowable under the regulations? - None of the other answers fall within one of the categories of allowable research. While these other studies have possible merit, there is no acceptable rationale for including prisoners. There is no benefit to the prisoners as individuals or as a group. A researcher is studying women recently admitted to a state prison. All potential subjects must have children under the age of five. Research subjects will be given a basket of toys to use at their children's first visit that the children can then take home. In assessing this proposal, the IRB needs to determine that the toys are Research with Prisoners SBE CITI Quiz_ 2023. A sociologist wants to study a culture that occurs in some women's prisons: "state families," in which individual prisoners take on certain roles within a group of like-minded prisoners. There is previous evidence that younger prisoners will use older inmates who play the roles of grandparents as a resource before they will turn to staff for help and advice. The lieutenant in charge of a dorm of long-term prisoners offers to gather volunteers to speak to the researcher and also offers to vouch for the integrity of the researcher. The use of this staff is: Neither Subpart C (Prisoners) nor Subpart D (Children) applies to juveniles in the correctional systems since Wardens of Juvenile prisoners - unlike those for adult prisoners - act in loco parentis for juvenile offenders. This statement is false because: A researcher is studying women recently admitted to a state prison. All potential subjects must have children under the age of five. Research subjects will be given a basket of toys to use at their children's first visit that the children can then take home. In assessing this proposal, the IRB needs to determine that the toys are: A researcher is examining the quality of life for prisoners who are HIV positive using surveys followed by interview. The IRB must ensure that: Which of the following statements about prison research is true? A researcher's study uses a dataset of prisoner demographic characteristics. This dataset includes criminal history data that predates incarceration and includes data on disciplinary behavior while in prison. There is no interaction with prisoners. The researcher claims and the IRB chair agrees that the study is exempt from IRB review. This decision: A researcher wants to contact former prisoners who are now on parole. She wants to study the difficulty of getting employment based on whether the subjects had been convicted of felony versus misdemeanor crimes. She needs to: One of your subjects is half way through a study of an investigational antidepressant that is injected weekly. The drug requires a taper-down regimen, that is, it should NOT be stopped abruptly. You learn that the subject will be admitted to prison next week prior to the next scheduled injection. What is the appropriate response for the researcher? Privacy and Confidentiality - SBE (Quiz/Answered) A researcher leaves a research file in her car while she attends a concert and her car is stolen. The file contains charts of aggregated numerical data from a research study with human subjects, but no other documents. The consent form said that no identifying information would be retained, and the researcher adhered to that component. Which of the following statements best characterizes what occurred? Although data encryption, using pseudonyms, and waiving documentation of consent, provide data protection, the researcher can link individuals to their responses. Therefore, the data are vulnerable to subpoena in civil, criminal, and administrative court proceedings. Unless the researcher has a Certificate of Confidentiality, he or she may be compelled to release individually identifiable information about research subjects. The Certificate provides the highest level of protection. Data are made anonymous by In a longitudinal study that will follow children from kindergarten through high school and will collect information about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable information? It is not possible for a researcher to promise confidentiality in a focus group setting. Participants may choose to repeat sensitive information outside the group setting even if they have signed confidentiality agreements. This is true even if the participants know each other. Using pseudonyms in the report does not remove concerns about what group members might say outside the group No agreements were made regarding confidentiality with the subjects who were unknowingly audio-taped; whose interactions were recorded, or whose cocaine use was revealed by another. Therefore, there was no breach of an agreement about confidentiality, but the subjects' right to decide who can access their personal information was violated. If a researcher told subjects that only he or she would have access to identifiable data and then gives identifiable data to others that action constitutes a breach of confidentiality and a violation of privacy - the subjects' right to control who has access to personal information. Reporting data in aggregate form, while protecting the identity of subjects, does not make the data anonymous. Keeping keys in secure locations and requiring members of the research team to sign confidentiality agreements are methods for protecting identifiable information but they do not involve destroying all identifying information so that the link between identity and data is gone forever. The only way to render data entirely anonymous is to remove all identifying information from the data and completely disconnect any links between the subjects and data about the subjects. The subjects' privacy has not been violated because the identity of subjects was not included in the file. The confidentiality of the data has not been breached because the data could not be linked to identifiers. Therefore, the correct answer is that there was neither a violation of privacy nor a breach of confidentiality. The release of aggregate data with no identifiers does not constitute a violation of privacy or a breach of confidentiality When a focus group deals with a potentially sensitive topic, which of the following statements about providing confidentiality to focus group participants is correct? - Which of the following constitutes both a breach of confidentiality (the research data have been disclosed, counter to the agreement between researcher and subjects) and a violation of subjects' privacy (the right of the individuals to be protected against intrusion into their personal lives or affairs)? CITI Privacy and Confidentiality SBE (Answered Quiz_ Spring 2023.) A researcher leaves a research file in her car while she attends a concert and her car is stolen. The file contains charts of aggregated numerical data from a research study with human subjects, but no other documents. The consent form said that no identifying information would be retained, and the researcher adhered to that component. Which of the following statements best characterizes what occurred? Which of the following constitutes both a breach of confidentiality (the research data have been disclosed, counter to the agreement between researcher and subjects) and a violation of subjects' privacy (the right of the individuals to be protected against intrusion into their personal lives or affairs)? In a longitudinal study that will follow children from kindergarten through high school and will collect information about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable information? When a focus group deals with a potentially sensitive topic, which of the following statements about providing confidentiality to focus group participants is correct? Data are made anonymous by IRB The Federal Regulations - SBE_ Quiz: questions (answered) According to the federal regulations, research is eligible for exemption, if: Which of the following statements about the relationship between an institution and the institution's IRB(s) is correct? According to federal regulations, the expedited review process may be used when the study procedures pose:. Continuing review of an approved and ongoing study posing more than minimal risk that was initially approved by a convened IRB: In addition to pregnant women, fetuses, and neonates, another subpart of the HHS regulations provides additional protections for which of the following vulnerable populations? The Federal Regulations – SBE Test. Which of the following are the three principles discussed in the Belmont Report - The history of ethical regulations in human subjects research began with the Where could student researchers and/or student subjects find additional resources regarding the IRB approval process? Which of the following elements must be included in an informed consent? Which type of IRB review does not require an IRB approval but does require a determination by the IRB or an IRB designee? How can faculty researchers avoid coercion of student subjects A student is conducting a research project that involves using a survey. The survey asks participants about their highest level of education, political affiliation, and views on various social issues. No identifiable information will be collected. This study would be categorized as which type of review? A student working on his dissertation plans on interviewing 15 principals in neighboring high schools. The student plans to collect data about the personal experiences the principals have had with disruptive students, what types of disciplinary actions were taken (including decisions they may have personally made), and their feelings or thoughts as to whether those actions were appropriate. Identifiers will be collected. This study would be categorized as which type of review? A master's degree candidate needs to conduct a research project for her master's thesis. She is interested in the types of junk food available to the public. She plans on going to the local convenience stores and asking the owners what types of junk food the store normally stock and which are the biggest sellers. Identifiers will not be collected. This study would fall under which of the following? Which of the following studies need IRB approval? What is the Institutional Review Board (IRB) charged with? (There may be more than one correct answer. Please be sure to select all correct answers.) Which of the following is an example of how the principle of beneficence is applied to a study involving human subjects? According to the Belmont Report, the moral requirement that there be fair outcomes in the selection of research subjects, expresses the principle of: The Belmont principle of beneficence requires that: The researcher's failure to protect research subjects from deductive disclosure is the primary ethical violation in which of the following studies? Humphreys collecting data for the Tearoom Trade study under the pretense that he was a lookout is an example of a violation of the principle of: According to the federal regulations, which of the following studies meets the definition of research with human subjects? A professor at Big State University is writing a biography about Bill Gates and conducting oral histories with all of Bill Gates' friends, family members and business acquaintances. The researcher submits the research proposal to the institution's IRB. What action can he expect by the IRB? According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, human subjects are living human beings about whom an investigator obtains data through interaction or intervention with the individual or: A medical record is an example of The primary purpose of a Certificate of Confidentiality is to Identify the example of when situation and time is key to assessing risk of harm in a research study: What statement about risks in social and behavioral sciences research is most accurate: Additional safeguards that may be included in a social and behavioral study may include: Risk of harm in social and behavioral sciences generally fall in three categories, which are: Which of the following statements about the relationship between an institution and the institution's IRB(s) is correct? According to the federal regulations, research is eligible for exemption, if Continuing review of an approved and ongoing study posing more than minimal risk that was initially approved by a convened IRB: According to federal regulations, the expedited review process may be used when the study procedures pose: In addition to pregnant women, fetuses, and neonates, another subpart of the DHHS regulations provides additional protections for which of the following vulnerable populations? A therapist at a free university clinic treats elementary school children with behavior problems who are referred by a social service agency. She is also a doctoral candidate who proposes using data she has and will collect about the children for a case-based research project. Which of the following statements about parental permission is correct? A general requirement for informed consent is that no informed consent may include any exculpatory language. Exculpatory language is that which waives or appears to waive any of the subject's legal rights or releases or appears to release those conducting the research from liability for negligence. Which of the following statements in a consent form is an example of exculpatory language? A criterion for waiving informed consent is that, when appropriate, subjects are provided additional pertinent information after the study. In which of the following studies would it NOT be appropriate to provide subjects with information about missing elements of consent: A waiver of the requirement for documentation of informed consent may be granted when: As part of the consent process, the federal regulations require researchers to A financial conflict of interest could involve A situation in which financial or other personal considerations have the potential to compromise or bias professional judgment and objectivity is an example of: Current NIH rules require investigators to disclose details regarding financial conflicts of interest to: Significant financial interest, as defined by the 2011 final rule that amended Public Health Service (PHS) regulations on Responsibility of Applicants for Promoting Objectivity in Research for which PHS Funding is Sought (42 C.F.R. Part 50, Subpart F) and Responsible Prospective Contractors (45 C.F.R. Part 94), include(s) (Check all that apply): The most important ethical concerns related to conflicts of interest in research are: - Data are made anonymous by Which of the following constitutes both a breach of confidentiality (the research data have been disclosed, counter to the agreement between researcher and subjects) and a violation of subjects' privacy (the right of the individuals to be protected against intrusion into their personal lives or affairs)? A researcher leaves a research file in her car while she attends a concert and her car is stolen. The file contains charts of aggregated numerical data from a research study with human subjects, but no other documents. The consent form said that no identifying information would be retained, and the researcher adhered to that component. Which of the following statements best characterizes what occurred? When a focus group deals with a potentially sensitive topic, which of the following statements about providing confidentiality to focus group participants is correct? In a longitudinal study that will follow children from kindergarten through high school and will collect information about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable information? A graduate student wants to examine the effect of print media versus televised media on individuals' position on several social issues. The superintendent of a local work release facility, a family friend, will allow the graduate student access to the prison population to help her quickly accrue subjects. The student's IRB should: A researcher is studying women recently admitted to a state prison. All potential subjects must have children under the age of five. Research subjects will be given a basket of toys to use at their children's first visit that the children can then take home. In assessing this proposal, the IRB needs to determine that the toys are: Which of the following statements about prison research is true? Which example of research with prisoners would be allowable under the regulations? - Neither Subpart C (Prisoners) nor Subpart D (Children) applies to juveniles in the correctional systems since Wardens of Juvenile prisoners When required, the information provided to the data subject in a HIPAA disclosure accounting... If you're unsure about the particulars of HIPAA research requirements at your organization or have questions, you can usually consult with: Under HIPAA, "retrospective research" (a.k.a., data mining) on collections of PHI generally ... HIPAA's protections for health information used for research purposes... A HIPAA authorization has which of the following characteristics: Vulnerable persons are those who are less able to protect themselves than other persons in a given situation. The Common Rule (45 CFR 46) has specific requirements for the following vulnerable populations, except: When a research project includes the collection of biological samples, all planned future uses of the samples, identifiers, and the data obtained from the samples, must be fully explained to the research subject. According to Subpart D, research with children may be eligible for exemption when: - A study that involves interviews of adults is eligible for expedited review. The researcher wants to add an adolescent population (aged 12 to 17) to the study and has designed a parental permission and assent process. No additional changes are planned. Which of the following statements about review of the revised protocol is accurate? The specific Department of Health and Human Services (DHHS) Regulations that apply to research with children are known as: A researcher asks an IRB to waive the requirement for parental permission for a study conducted in schools because the nature of the research requires participation of all the children present in classrooms on the day the research will take place. Assuming that the basic research design could be approved by the IRB and the school, which of the following requirements must be met before an IRB could waive parental permission? - In addition to the general provisions of the Common Rule (the federal regulations for protecting research subjects), the following regulations also govern research in the public schools: Which of the following types of information may schools disclose without consent from the parent or student to a researcher at a local university? Which federal regulation or law governs how researchers can obtain data about subjects' disciplinary status in school from academic records? Defining Research with Human Subjects - SBE Complete Test_2022/2023. Which of the following is an example of how the principle of beneficence is applied to a study involving human subjects? The researcher's failure to protect research subjects from deductive disclosure is the primary ethical violation in which of the following studies? Which of the following studies is linked most directly to the establishment of the National Research Act in 1974 and ultimately to the Belmont Report and Federal regulations for human subject protection? The Belmont principle of beneficence requires that: According to the Belmont Report, the moral requirement that there be fair outcomes in the selection of research subjects, expresses the principle of: According to the federal regulations, human subjects are living individuals about whom an investigator conducting research obtains information through interaction or intervention with the individual, and uses, studies, or analyzes the information; or: - According to the federal regulations, which of the following studies meets the definition of research with human subjects?. Census data (the final report as published by the Census Bureau) is an example of: - According to the federal regulations, which of the following studies meets the definition of research with human subjects? A medical record is an example of: A professor at Big State University is writing a biography about Bill Gates and conducting oral histories with all of Bill Gates' friends, family members and business acquaintances. The researcher submits the research proposal to the institution's IRB. What action can he expect by the IRB? According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which of the following studies meets the definition of research with human subjects? A subject in a clinical research trial experiences a serious, unanticipated adverse drug experience. How should the investigator proceed, with respect to the IRB, after the discovery of the adverse event occurrence? How long is an investigator required to keep consent documents, IRB correspondence, and research records? According to federal regulations, which of the following best describes when expedited review of a new, proposed study may be used by the IRB? Amendments involving changes to IRB approved protocols do NOT need prior IRB approval if: IRB continuing review of an approved protocol must: A therapist at a free university clinic treats elementary school children with behavior problems who are referred by a social service agency. She is also a doctoral candidate who proposes using data she has and will collect about the children for a case-based research project. Which of the following statements about parental permission is correct? A general requirement for informed consent is that no informed consent may include any exculpatory language. Exculpatory language is that which waives or appears to waive any of the subject's legal rights or releases or appears to release those conducting the research from liability for negligence. Which of the following statements in a consent form is an example of exculpatory language? A criterion for waiving informed consent is that, when appropriate, subjects are provided additional pertinent information after the study. In which of the following studies would it NOT be appropriate to provide subjects with information about missing elements of consent: A waiver of the requirement for documentation of informed consent may be granted when: As part of the consent process, the federal regulations require researchers to: - Which of the following constitutes both a breach of confidentiality (the research data have been disclosed, counter to the agreement between researcher and subjects) and a violation of subjects' privacy (the right of the individuals to be protected against intrusion into their personal lives or affairs)? Data are made anonymous by When a focus group deals with a potentially sensitive topic, which of the following statements about providing confidentiality to focus group participants is correct? - In a longitudinal study that will follow children from kindergarten through high school and will collect information about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable information? A researcher leaves a research file in her car while she attends a concert and her car is stolen. The file contains charts of aggregated numerical data from a research study with human subjects, but no other documents. The consent form said that no identifying information would be retained, and the researcher adhered to that component. Which of the following statements best characterizes what occurred? Which of the following considerations was relevant to the IRB's determination that this activity does not constitute research with human subjects? As part of a research study, a physician plans to review medical records of the next 50 of her patients who require magnetic resonance imaging (MRI) scans for clinical treatment to explore factors related to patients requiring MRI scans. The physician will review the medical records, and write down the clinical indication for the scans, any existing injuries, current prescriptions, as well as other clinical data. The clinical indication for the scans and the other clinical data will be collected in the medical records for treatment purposes as part of standard clinical care. The physician will use a coding system to be able to identify the patient's information; however, the "key" to the coding system will be stored separately from the data in a locked cabinet that only she will have access to. Which of the following is true? An investigator obtains consent from subjects to review their medical records and HIV status. He plans to go back to the medical record, so the HIV status information is stored along with patient identifiers in a database that he keeps on his laptop computer. His laptop is stolen. This incident constitutes: NBAC proposed a concept of vulnerability in research based on features of potential subjects or of their situation. Which of the following was NOT included as possibly leading to vulnerability? n considering NBAC's analytic approach, an otherwise competent person who is acutely ill might be considered at especially high risk of harm for: According to the authors, there are four common abuses that historically are described as giving rise to vulnerability . Which response below contains the correct four? - Identify the following groups that are protected in the federal regulations (45 CFR 46), specifically in Subparts B, C and D with additional protections Which is true of inducements in research? Defining Research with Human Subjects - SBE CITI (QUIZ) A professor at Big State University is writing a biography about Bill Gates and conducting oral histories with all of Bill Gates' friends, family members and business acquaintances. The researcher submits the research proposal to the institution's IRB. What action can he expect by the IRB? According to the federal regulations, human subjects are living human beings about whom an investigator obtains data through interaction or intervention with the individual or: According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which of the following studies meets the definition of research with human subjects? Census data is an example of: CITI History and Ethical Principles - SBE Which of the following studies is linked most directly to the establishment of the National Research Act in 1974 and ultimately to the Belmont Report and federal regulations for human subject protection? - Humphreys' collecting data for the Tearoom Trade study under the pretense that he was a lookout is an example of a violation of the principle of: An example cited in the Belmont Report (The National Commission 1979) stated that "During the 19th and early 20th centuries the burdens of serving as research subjects fell largely upon poor ward patients, while the benefits of improved medical care flowed primarily to private patients." This is an example of a violation of which Belmont principle? A study was submitted to the IRB designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. The IRB approved the study and consent form. The consent form includes all the required information. The use of a consent form is an example of the Belmont principle of: The Belmont principle of beneficence requires that: The researcher's failure to protect research subjects from deductive disclosure of identity (that is, the re-identification of subjects by other researchers) is the primary ethical violation in which of the following studies? History and Ethical Principles - SBE CITI Training questions Which of the following are the three principles discussed in the belmont report? Which of the following is an example of how the principle of beneficence can be applied to a study employing human subjects? All of the following are true regarding the belmont report except:. The history of ethical regulations in human subjects research began with... Where could student researchers and/or student subjects find additional resources regarding the IRB approval process? Which element is to be included in informed consent? Which type of IRB review does not require an IRB approval but does require a determination by the IRB or and IRB designee How can faculty researchers avoid coercion of student subjects? A student is conducting a research project that involves using a survey. it asks participants about their highest level of education, political affiliation, and views on various social issues. No identifiable info will be collected. What is this? A student working on his dissertation plans on interviewing 15 principles in neighboring high schools. The student will collect data about personal experiences they had with disruptive students, disciplinary action and feelings/thoughts. Identifiers will be collected. What is this? A masters degree candidate needs to conduct a research project for her masters thesis. She is interested in junk food available to public. She plans on going to the grocery stores and asking for highest sellers. Identifiers will not be collected. What is this? - Which of the following studies needs IRB approval? What is the IRB charged with? Which of the following is an example of how the principle of beneficence is applied to a study involving human subjects? Which of the following studies is linked most directly to the est. of the national research act in 1974 and ultimately to the belmont report and federal regulations for human subject protection? the researchers failure to protect subjects from deductive disclosure is the primary ethical violation in which of the following studies? The belmont principle of beneficence requires that... According to federal regulations, which of the following studies meets the definition of research with human subjects? According to the federal regulations, which meets criteria for human subjects? Researcher gains info through... which of the following statements about the relationship between an institution and an institutions IRB is correct? According to federal regulations, research is eligible for exemption if.. Continuing review of an approval and ongoing protocol... DHHS regulations provides protection for... Expedited review process may be used when the study procedures pose.. What statement about risks in social and behavioral sciences research in most accurate? If disclosure of a subjects involvement is risky and the consent form is the only one thing linking the subjects... certificate of confidentiality? Risk of harm should be evaluated by... Studying coping with adults who experienced abused as a child. There will be a consent form, the most likely additional risk is... A therapist at a free university clinic treats elementary school children with behavior problems who are referred by a social service agency. She is also a doctoral candidate who proposes using data she has and will collect about the children for s case based research project. Which of the following research statements about parental permission is correct? A general requirement for informed consent is that no informed consent may include any exculpatory language. Waives or appears to wave the subjects leg l rights or releases or appears to release those conducting the research from liability from negligence. Which is an example of exculpatory language? A criterion for waiving informed consent is that, when appropriate, subjects are provided additional pertinent info after the study. In which of the following studies would it not be appropriate to provide subjects with info about missing elements of consent: A waiver of the requirement for documentation of informed consent may be granted when... As part of the consent process, the federal regulations require some researchers to... - In a longitudinal study that will follow children from K to HS and collect info about illegal activities, which of the following confidentiality procedures would protect against compelled disclosure of individually identifiable info? When a focus group deals with a potentially sensitive topic, providing confidentiality means: Data are made anonymous by... Which constitutes a breach of confidentiality and a violation of subjects privacy? A researchers data is stolen from her car. Files contained aggregated data. Consent form said no identifying info would be retained ad researchers adhered A researcher is examining the quality of life for prisoners who are HIV positive using surveys followed b interviews. The IRB must ensure that: If a grad student speaks to a super (family friend)for a prisoner population. This students IRB should... A researchers uses a data set of prisoner demographic characteristics/ No interaction with prisoners. The IRB chair agrees that the study is exempt from IRB review. [Show More]
Last updated: 1 year ago
Preview 1 out of 41 pages
.png)
Reviews( 0 )
Recommended For You
Business> QUESTIONS & ANSWERS > CLM 031 EXAM (All)

CLM 031 EXAM
CLM 031 = 100% Question 1: 5b Select the statement that is correct concerning performance work statement (PWS) requirements: - All answers are correct. - PWS should describe requirements necessary...
By Book Worm, Certified , Uploaded: Nov 03, 2022
$5
*NURSING> QUESTIONS & ANSWERS > PHIL 347 Week 6 Checkpoint Quiz. Score 100/100 (All)

PHIL 347 Week 6 Checkpoint Quiz. Score 100/100
Question: What are the three fundamental reasoning strategies listed in the text? Question: What is comparative reasoning? On what skill is it based? Question: We learned four tests for evaluating...
By Amanda Rosales , Uploaded: Mar 24, 2021
$7
Business> QUESTIONS & ANSWERS > BUSINESS 1007 (All)

BUSINESS 1007
BUSINESS 1007 07 Key 1. (p. 178) Managers utilize organizational resources such as employees, information, and equipment to accomplish goals. 2. (p. 178) The main job of managers today is to w...
By Kirsch , Uploaded: Oct 19, 2019
$6
Anthropology> QUESTIONS & ANSWERS > KOR 352 FA19 101 week 8 Quiz. Already Graded A (All)

KOR 352 FA19 101 week 8 Quiz. Already Graded A
KOR 352 FA19 101: Week 8 Quiz Question 1 (0.25 points) Which of the following is not true of Kim and Finch’s observations during their field research in South Korea from 1997 to 2000? Question 1 o...
By Kirsch , Uploaded: Oct 17, 2019
$9
E-Commerce> QUESTIONS & ANSWERS > ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score (All)
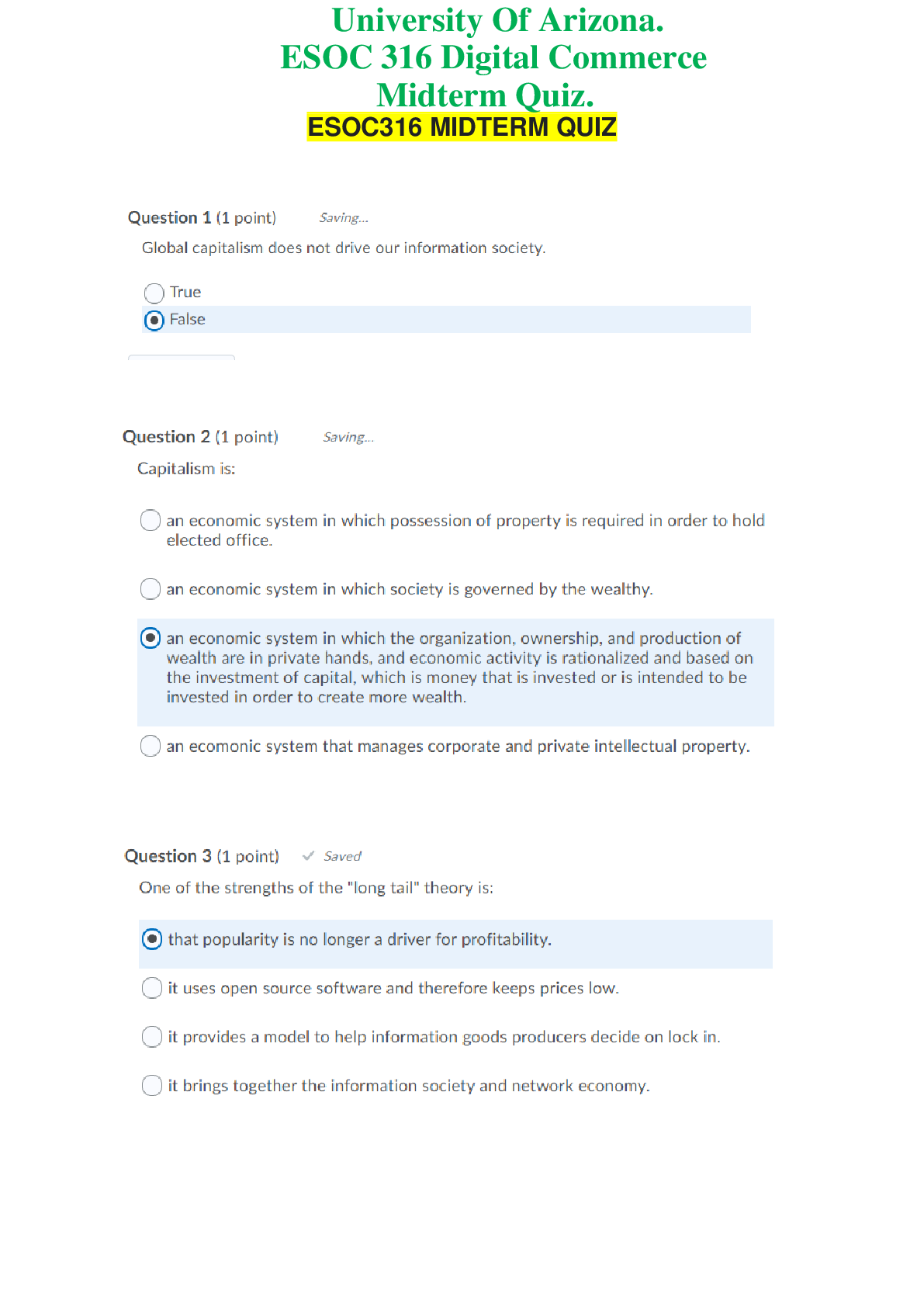
ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score
ESOC 316 Digital Commerce - University Of Arizona. Midterm Quiz. 20 Q&A. 100% Score ESOC316 MIDTERM QUIZQuestion 6 (1 point) Saved Information has several properties that make information goods...
By Kirsch , Uploaded: Oct 15, 2019
$9.5
Marketing> QUESTIONS & ANSWERS > Marketing Management Chapter 2 to Chapter 10 Q&A (All)

Marketing Management Chapter 2 to Chapter 10 Q&A
Chapter 2 to Chapter 10 Chapter 2: Developing Marketing Strategies and Plans GENERAL CONCEPT QUESTIONS Multiple Choice 66 Chapter 1: Marketing: Managing Profitable Customer Relationships...
By Kirsch , Uploaded: Oct 14, 2019
$10
Marketing> QUESTIONS & ANSWERS > MKT 530 Customer Relationship Management. 155 Questions and Answers (All)

MKT 530 Customer Relationship Management. 155 Questions and Answers
MKT 530 All Questions and Answers MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) As the manager of an organization that is att...
By Kirsch , Uploaded: Oct 14, 2019
$10
Art> QUESTIONS & ANSWERS > MAS 337 Exam 1. Graded A (All)

MAS 337 Exam 1. Graded A
MAS 337 Exam 1 Match the son with its corresponding region. 1.Oaxaca 2.Veracruz 3.Michoacan 4.Jalisco 5.Hidalgo 1. Son istemeno 2. Son Jarocho 3. Son Abajeno 4. Son Jalisciense 5. Son Huast...
By Kirsch , Uploaded: Oct 14, 2019
$6
Art> QUESTIONS & ANSWERS > MUS 337 Final Exam Graded 100%. (All)

MUS 337 Final Exam Graded 100%.
MUS 337 Final Daisy Moreno-Cota Fri Dec 11 12:59:42 PST 2015 Tone or aural color Volume and articulation of sounds Frequency of the tone Phonic structure or relationships between the sounds Dura...
By Kirsch , Uploaded: Oct 12, 2019
$9
Art> QUESTIONS & ANSWERS > MUS 337 Final. Graded 100%. (All)

MUS 337 Final. Graded 100%.
MUS 337 Final Daisy Moreno-Cota Fri Dec 11 12:59:42 PST 2015 Tone or aural color Volume and articulation of sounds Frequency of the tone Phonic structure or relationships between the sounds Dura...
By Kirsch , Uploaded: Oct 12, 2019
$9
Document information
Connected school, study & course
About the document
Uploaded On
Jan 27, 2023
Number of pages
41
Written in
Additional information
This document has been written for:
Uploaded
Jan 27, 2023
Downloads
0
Views
264






