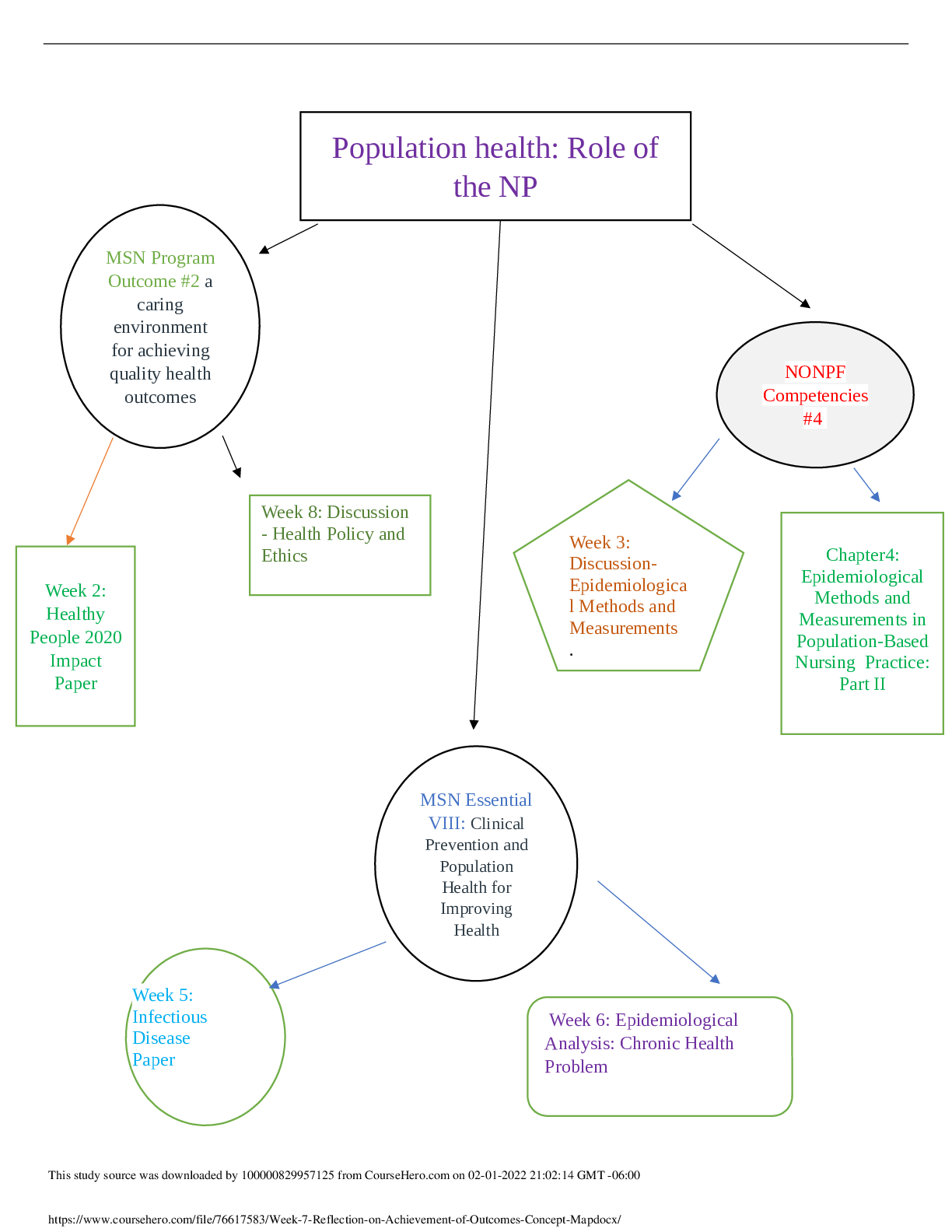
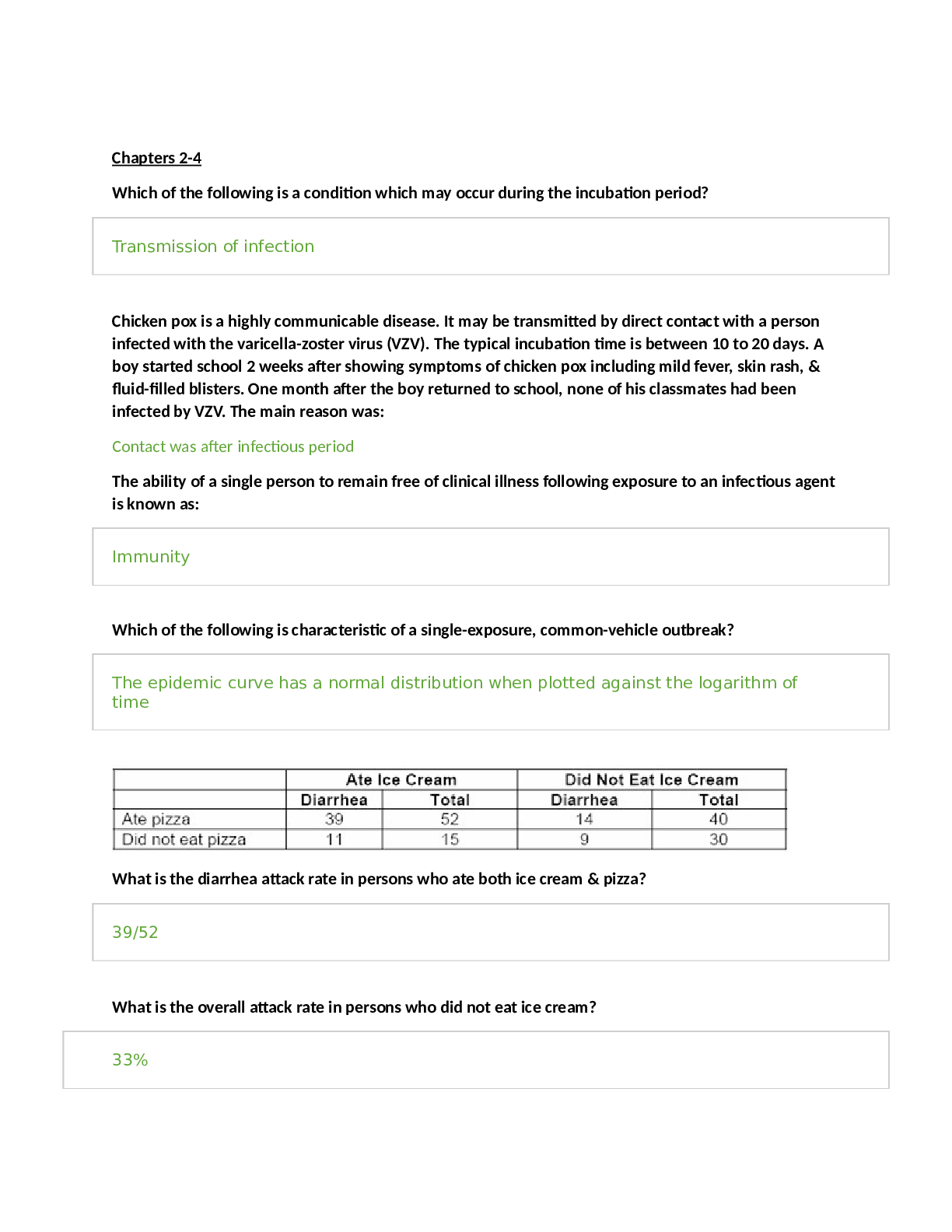
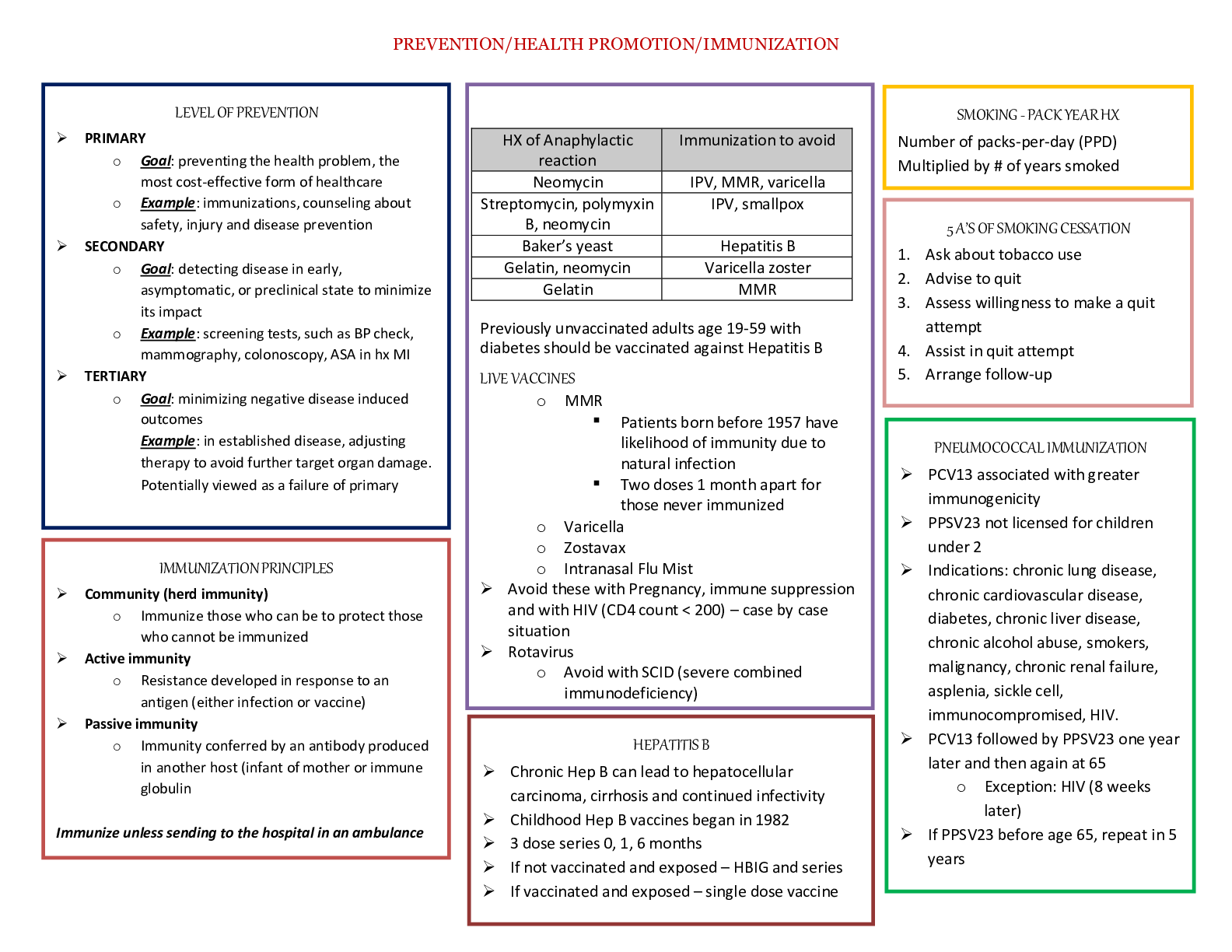
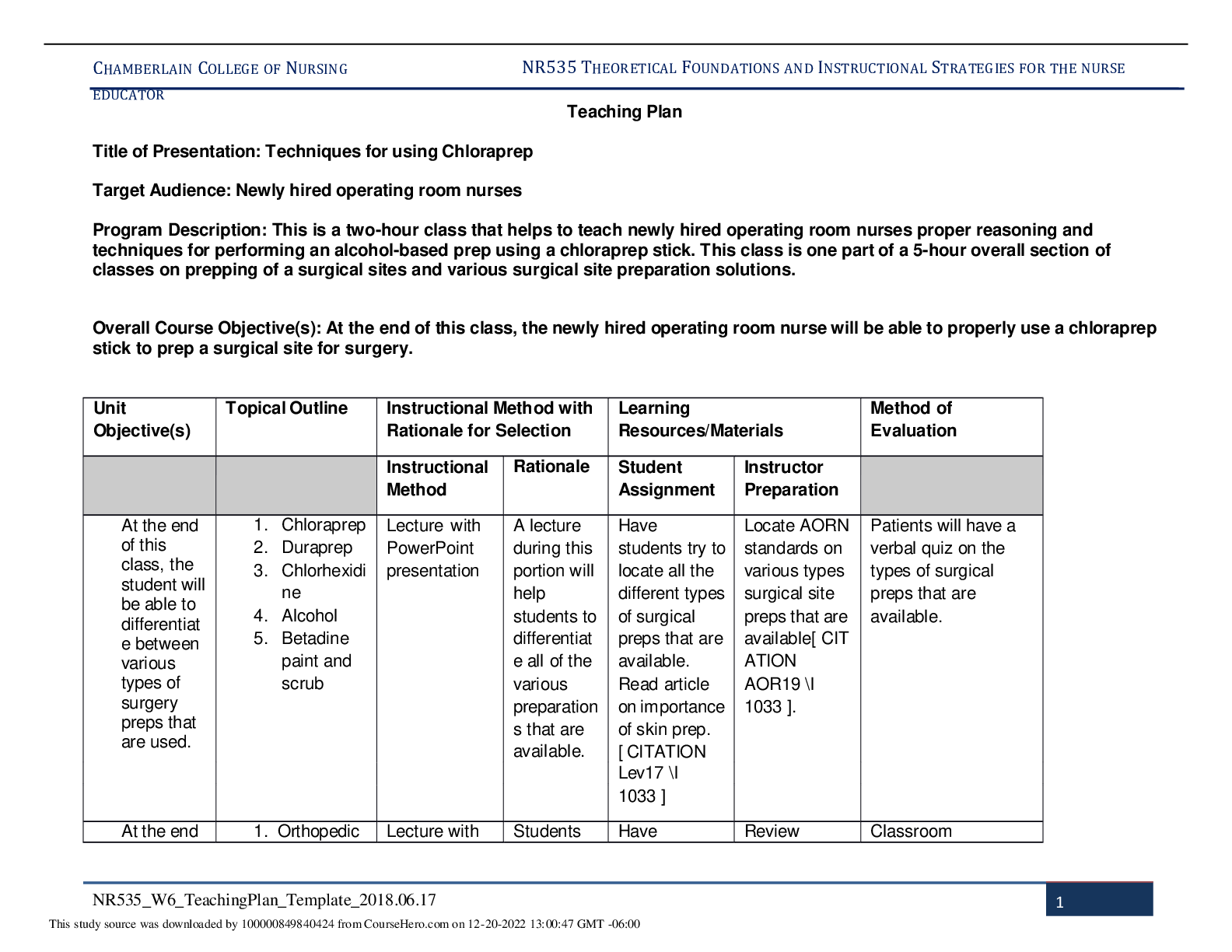
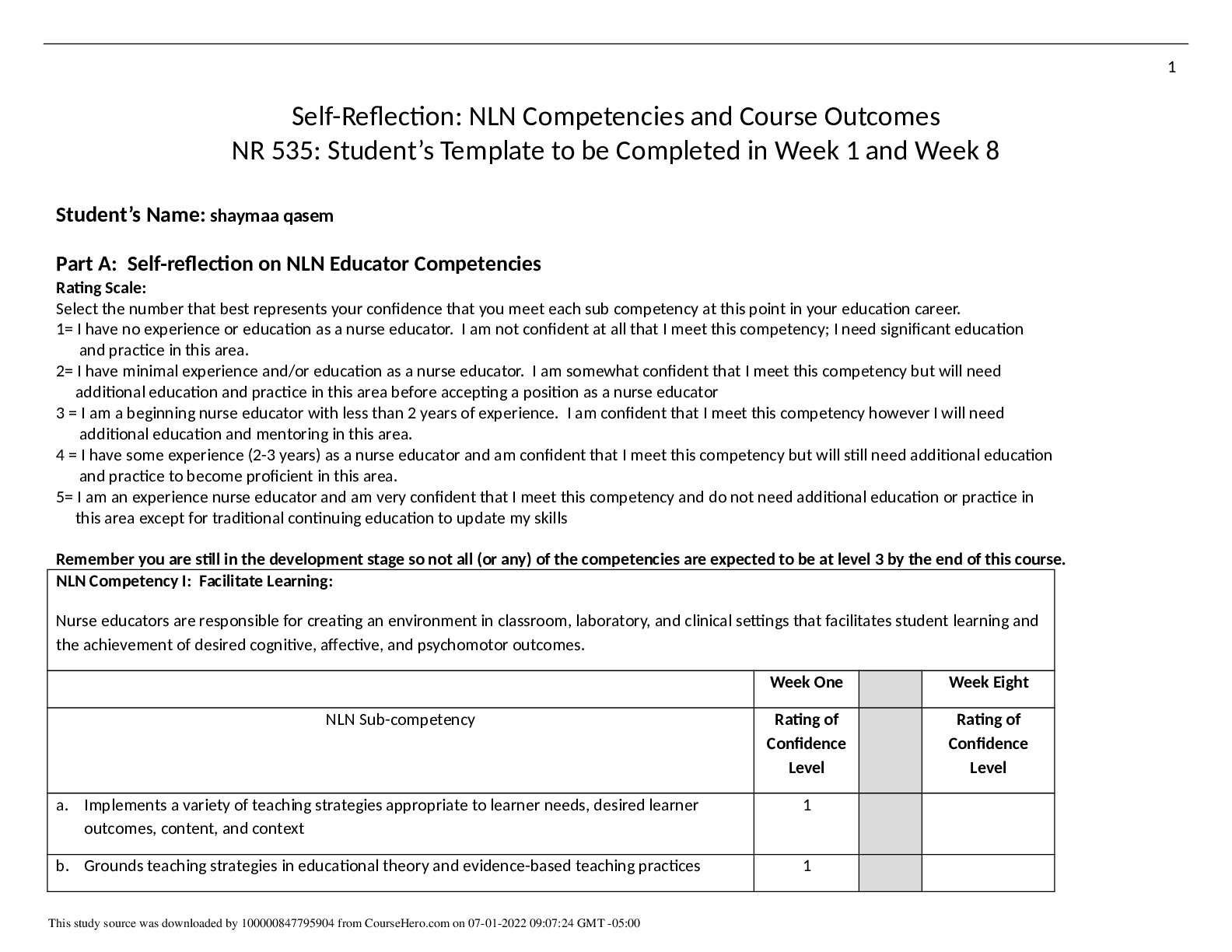
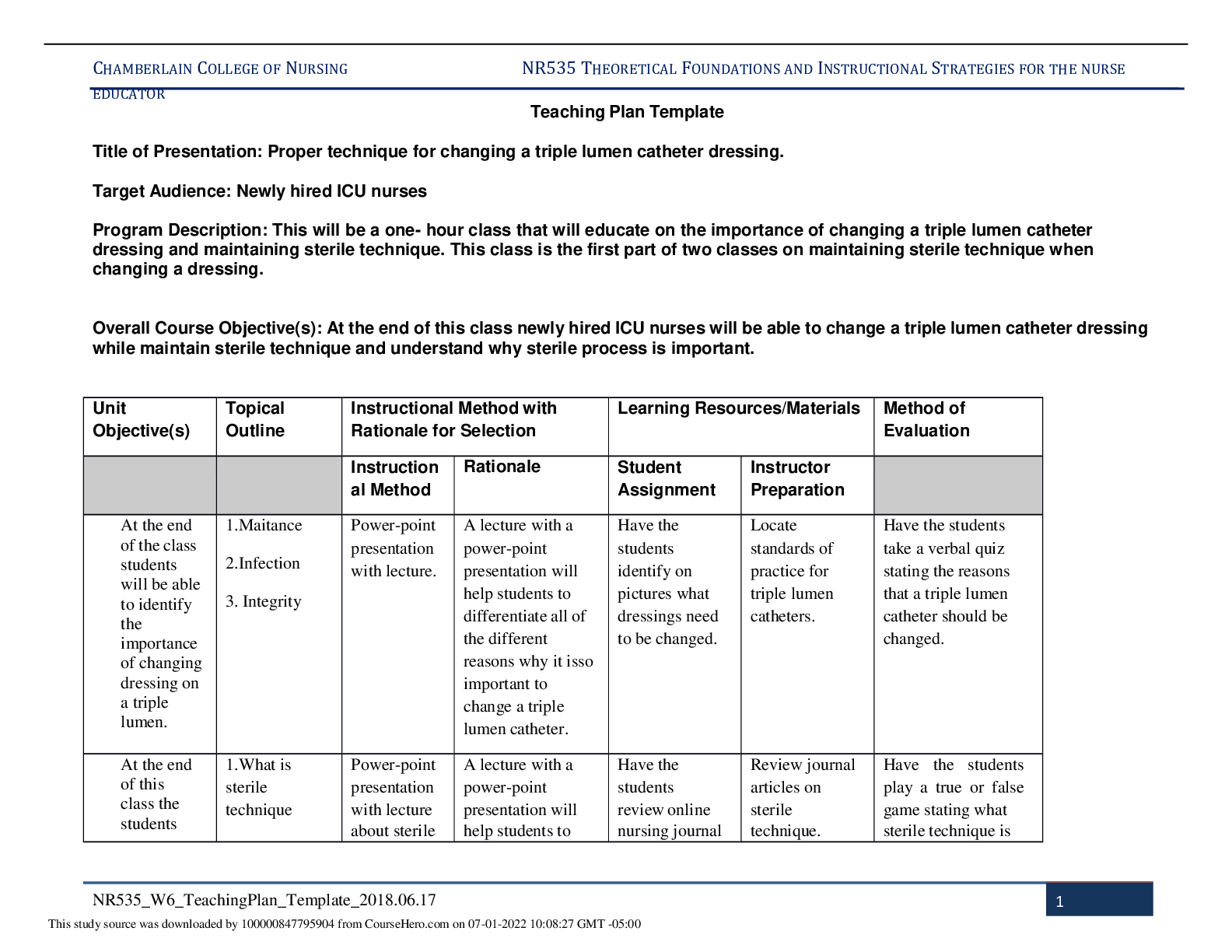
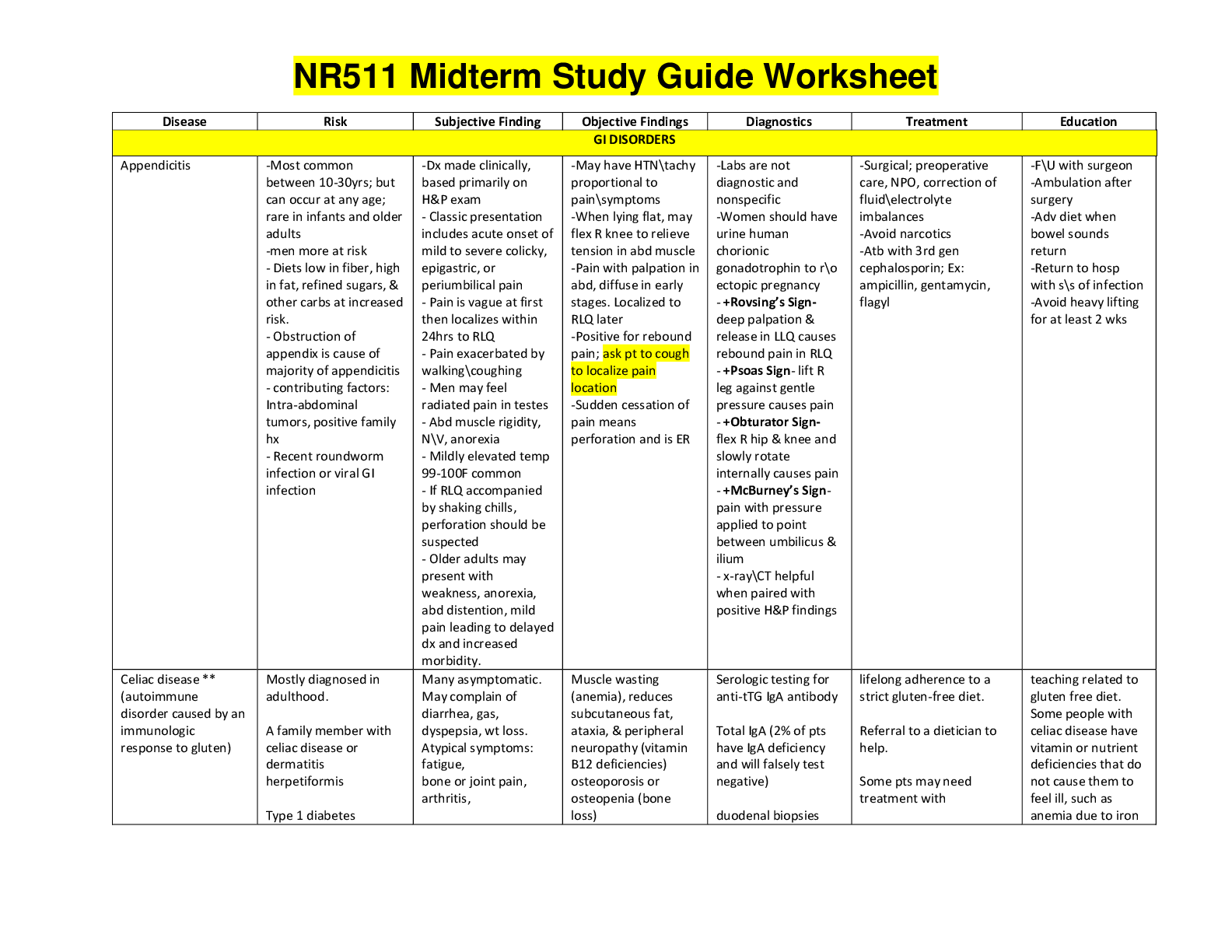
Pharmacology > STUDY GUIDE > NR 565 Week 4 MidTerm Review CHAPTER 15 DRUGS AFFECTING THE CNS Study_Guide | Chamberlain College of (All)
NR 565 Week 4 MidTerm Review CHAPTER 15 DRUGS AFFECTING THE CNS Study_Guide | Chamberlain College of Nursing
Document Content and Description Below
Chapter 15: Drugs Affecting the Central Nervous System Anorexiants (p. 226): Short-term adjuncts to calorie limiting, cognitive-behavioral, weight-loss programs for severely obese individuals. Nona... mphetamine appetite suppressants are commonly used today but are chemically and pharmacologically related to amphetamines. (Phendimetrazine, Benzphetamine, Diethylpropion HCl, Phentermine, Lorcaserin) Precautions and Contraindications: High risk of tolerance and dependence. Should be used in caution with patients who have a history of alcohol or drug dependence. Use should be limited to 6 months and discontinued at any sign of tolerance. Substance Abuse: Patients who abuse substances such as cocaine, phencyclidine, and methamphetamine should not be prescribed anorexiants because of the potential for excessive adrenergic stimulation. Alcoholics: Actively drinking alcoholics taking anorexiants have experienced depression, paranoia, and psychosis. Diabetes: Patients with diabetes may experiences altered insulin or oral hypoglycemic dosage requirements. Lorcaserin: Serotonergic drug. Patients may develop serotonin syndrome or Neuroleptic Malignant Syndrome like reactions if coadministered with serotonergic drugs. Pregnancy Category X and is not approved in children under 18. Anticonvulsants (p. 227): Hydantoins: First line treatment of choice for tonic-clonic and partial complex seizures and the lease sedating drugs used to treat seizure disorders of any type. (phenytoin- Dilantin, ethotoin-Peganone, fosphenytoin-Cerebyx). Pharmacodynamics: Inhibit and stabilize electrical discharges in the motor cortex of the brain by affecting the influx of sodium ions into the neuron during depolarization and repolarization, slowing the propagation and spread of abnormal discharges. Metabolism and Excretion: Metabolism takes place in the liver and excretion via the kidneys. Plasma half-lives range from 6-24 hours. Precautions and Contraindications: Contraindicated under conditions of hypersensitivity. Phenytoin induced hepatitis is a common hypersensitivity reaction. Other hypersensitivity reactions include fever, rash, arthralgias, and lymphadenopathy. Phenytoin: May cause severe cardiovascular events and death has resulted from too-rapid IV administration. Phenytoin has a Black-Box Warning that IV administration should not exceed 50mg/minute in adults and 1-3 mg/kg/minute in pediatric patients owing to risk of cardiovascular reactions associated with a too rapid rate of administration. Contraindicated in sinus bradycardia, sinoatrial block, second-and third- degree AV block, and Stokes-Adams syndrome. Should be used cautiously in patients with hepatic or renal disease. Ethotoin: Contraindicated in the presence of hepatic or hematological disorders. Fetal Defects: Pregnancy Category D. About 10% of babies have defects in Mother’s who take phenytoin during pregnancy. Newborns exposed to phenytoin is utero may experience decreased levels of Vitamin K- dependent clotting factors and the mother should receive Vitamin K before delivery and the newborn at birth. Adverse Drug Reactions: CNS effects (agitation, ataxia, confusion, dizziness, drowsiness, headache, and nystagmus), Cardiovascular effects (hypotension, tachycardia, atrial and ventricular conduction depression, and ventricular fibrillation), GI effects (nausea, vomiting, anorexia, altered taste, constipation, dry mouth, and gingival hyperplasia), GU effects (urinary retention and reddish- brown discoloration of urine), Dermatologic reactions (Stevens-Johnson Syndrome and toxic epidermal necrolysis). Drug Interactions: Interactions that increase hydantoins effect because of increased metabolism, competition for binding sites or for unknown reasons occur with benzodiazepines, cimetidine, disulfiram, TCAs, salicylates, and valproic acid. Interactions that decrease hydantoin’s effect include barbiturates, rifampin, theophylline, influenza vaccine, pyridoxine, and antacids. Oral contraceptives effect is decreased with use of hydantoins. Acute alcohol intake may increase phenytoin serum levels, whereas chronic alcohol use may decrease levels. IV phenytoin should only be mixed with normal saline. Monitoring: Patients should be assessed for phenytoin hypersensitivity syndrome (fever, skin rash, lymphadenopathy), which usually occurs at 3-8 weeks. Baseline CBC, urinalysis, and LFTs should be assessed prior to onset of treatment, with frequent reassessment during the first few months of treatment. Plasma levels should be monitored, especially when drugs that increase plasma hydantoin, such as ibuprofen, are used. Patient Education: Abrupt withdrawal may lead to status epilepticus. Advise the patient to wear a medical identification bracelet, to avoid hazardous situations if drowsiness occurs, and to report adverse effects to the clinician. Patients should avoid alcohol use. Maintain good oral hygiene to prevent tenderness, bleeding, and gingival hyperplasia. Phenytoin may color the urine red, pink, or reddish brown but the color change is not a cause for alarm. Advise diabetic patients to monitor blood glucose levels and report significant changes to the clinician. Iminostilbenes (p. 235): (Carbamazepine-Tegretol and oxcarbazepine-Trileptal). Structurally related to TCAs. Used to treat epilepsy, bipolar affective disorder, aggressive and assaultive behavior, and some neuralgias. Pharmacodynamics: Thought to affect the sodium channels, slowing influx of sodium in the cortical neurons and slowing the spread of abnormal activity. Carbamazepine exerts its effect by depressing transmission in the nucleus ventralis anterior of the thalamus. This area is associated with the spread of seizure discharge. Metabolism and Excretion: Carbamazepine is metabolized in the liver and has the unique ability to induce its own metabolism (autoinduction). Due to autoinduction, initial concentrations within a therapeutic range may later fall despite good compliance. It also induces the metabolism of many CYP450 enzymes and other substrates. Excretion is through urine and feces. Oxcarbazepine is metabolized into an active metabolite 10- monohydroxy metabolite, which is responsible for the pharmacologic effect of the drug. The metabolites of oxcarbazepine are excreted 95% in urine, 4% in feces, and 1% unmetabolized oxcarbazepine. Precautions and Contraindications: Carbamazepine: Contraindications include hypersensitivity to carbamazepine or TCAs, history of bone marrow suppression, and current administration with MAOIs. Carbamazepine is Pregnancy Category D; tetratogenic defects have occurred including spina bifida. Black-Box Warning regarding serious dermatological reactions, particularly among patients of Asian ethnicity (Stevens-Johnson Syndrome, toxic epidermal necrolysis and risk of developing aplastic anemia and agranulocytosis). Patients of Asian ethnicity should be screened for presence of the HLA- B*1502 genetic variant prior to starting carbamazepine. Caution is advised in patients with a history of previous adverse hematological reactions to any drugs and in those with cardiac, renal, or hepatic impairment. Oxcarbazepine: Pregnancy Category C; it crosses the placenta and adverse effects have been noted in animal studies. Contraindicated with hypersensitivity to oxcarbazepine. Adverse Drug Reactions: Carbamazepine has a Black Box Warning regarding the development of Stevens-Johnson Syndrome and toxic epidermal necrolysis in patients of Asian ethnicity. Carbamazepine has a Black Box Warning due to its potential to cause blood dyscrasias, some potentially lethal. Carbamazepine can depress the bone marrow and lead to leukopenia, thrombocytopenia, agranulocytosis, and aplastic anemia. For that reason, a baseline CBC, chemistry, LFTs, and TSH should be obtained, followed by periodic monitoring. Follow up studies should be more frequent initially, decreasing to every 3-4 months if the results remain normal. Other adverse reactions to carbamazepine include hepatic damage and impaired thyroid function. Less serious adverse events include drowsiness, dizziness, blurred vision, ataxia, nausea, vomiting, dry mouth, diplopia, and headache. The most common adverse effects observed in patients taking oxcarbazepine were dizziness, diplopia, somnolence, fatigue, N/V, ataxia, abdominal pain, tremor, and dyspepsia. Hyponatremia may occur, particularly in the first 3 months of therapy. Drug Interactions: Carbamazepine: The interactions of most significance are those that increase the plasma level of carbamazepine to potentially toxic levels, such as the concurrent administration of propoxyphene, hydantoins, cimetidine, some Abx (erythromycin, clarithromycin), isoniazid, and verapamil. Interactions that decrease plasma levels of the other drug occur with beta blockers, succinimides, valproic acid, warfarin, haloperidol, doxycycline, and nondepolarizing muscle relaxants. Grapefruit juice increases serum levels and effects of carbamazepine. Oxcarbazepine: Can inhibit CYP2C19 and induce CYP3A4/5, leading to increased levels of drugs metabolized by CYP2C19. May decrease effectiveness of contraceptives containing ethinylestradiol and levonorgestrel. Monitoring: Patients should be monitored for seizure activity, severity, and duration. Adverse effects should be monitored, including CNS depression and suicidality or behavioral changes. Plasma carbamazepine levels should be monitored on a regular basis. Therapeutic range is 4-12 mcg/mL. Children and elderly patients may develop toxicity (HTN, tachycardia, ECG changes, stupor, agitation, nystagmus, urinary retention, respiratory depression, seizures, and coma) at levels below 12. Oxcarbazepine does not require serum level monitoring. Serum sodium levels should be monitored for the first 3 months of therapy, especially if the patient is taking other drugs that may cause hyponatremia. Patient Education: Patients taking carbamazepine should be instructed to report any symptoms such as skin lesions, bruising, fever, or sore throat. Administration with food may increase absorption, and because carbamazepine can be sedating, care should be exercised in situations in which mental and physical alertness is required for safety. Oxcarbazepine may cause hyponatremia; therefore, patients should be educated regarding symptoms of hyponatremia which include nausea, fatigue, headache, confusion, and increased seizures. Patients should report swelling of face, eyes, lips, or tongue, which may be symptoms of Stevens- Johnson Syndrome. Patients should report any mood changes or suicidal thoughts. Succinimides: Used to treat absence seizures (ethosuximide-Zarontin and methsuximide- Celontin). Pharmacodynamics: Decrease nerve impulses and transmission in the motor cortex. This produces a variety of effects, including an increase in the seizure threshold and reducing the EEG spike and wave pattern of absence seizures. Metabolism and Excretion: Metabolized in the liver and excreted through the urinary tract, although a small amount of phensuximide is excreted in bile. Precautions and Contraindications: With careful monitoring of plasma levels, appear to be safe for use during pregnancy and are Pregnancy Category C. Contraindicated during lactation. Can cause blood dyscrasias and use should be preceded by a CBC with differential repeated at frequent intervals initially and less often as the patient continues on the medication without adverse effects. LFTs should also be obtained prior to instituting treatment. Adverse Drug Reactions: Most common are GI distress, which can be relieved by taking the medication with food or milk, and CNS depression (sedation, ataxia, and lethargy). Other adverse reactions include headache, rash, pruritus, suicidal thoughts, and mood changes. Cases of systemic lupus erythematosus have also been reported. Drug Interactions: The most significant drug interactions are those that increase CNS depression, such as alcohol, opioid agonists, benzodiazepines, and CNS depressants. May be given with other anticonvulsants but may antagonize them and contribute to tonic-myoclonic breakthrough seizures, therefore requiring the need for a higher dose of the other anticonvulsant. Avoid concurrent use with TCAs and phenothiazines because an antagonistic effect on succinimides may lower the patient’s seizure threshold. Haloperidol may change the pattern or frequency of seizures, necessitating an adjustment in dosage of the anticonvulsant. Succinimides may decrease the effectives of oral contraceptives. Monitoring: Plasma levels should be monitored. Normal therapeutic range of ethosuximide is 40-100 mcg/mL; levels over 150 are considered toxic. The therapeutic range for methsuximide is 10-40 mcg/mL, with levels over 40 considered toxic. Should also monitor seizure activity, evaluate liver, renal, and hematological studies periodically for adverse effects. Patient Education: Advise the patient to avoid alcohol and, if sedation occurs, to avoid hazardous activities. Advise the client to report any behavioral changes to the clinician. Caution the client that withdrawal of the medication may precipitate absence seizures. Inform the client taking phensuximide that harmless changes in urine color may occur. Drugs that Affect GABA (p. 238) : The anti-epileptic drugs that affect GABA include benzodiazepines, gabapentin, topiramate, and tiagabine. The AEDs that affect the inhibitory neurotransmitter GABA are also used for pain, including neuropathic pain (gabapentin) and migraine (topiramate). Pharmacodynamics: Gabapentin is thought to be a GABA analogue that binds to unknown receptors in the brain; it does not bind to GABA receptors, nor does it mimic GABA. Topiramate may block sodium channels or potentiate GABA. Tiagabine may potentiate the action of GABA by blocking GABA reuptake into presynaptic neurons, allowing for more GABA to be available to bind to postsynaptic neuronal receptors. Precautions and Contraindications: Gabapentin: Should not be abruptly discontinued because it may precipitate status epilepticus; the dose should be decreased over at least a week. Increase the risk of suicidal behavior and ideation; patients should be monitored for emergence or worsening of depression, suicidal thoughts, or changes in behavior. Pregnancy Category C. Excreted in breast milk. Should be used in nursing women only if the benefits outweigh the risks. Topiramate: May have decreased concentrations of serum bicarbonate due to inhibition of carbonic anhydrase and increased renal bicarbonate loss, leading to a hyperchloremic metabolic acidosis. Serum bicarbonate should be monitored at baseline and periodically throughout therapy. An ocular syndrome consisting of acute myopia and angle closure glaucoma has been reported in adults and children taking topiramate. Patients who have eye pain or blurred vision should contact their provider immediately. A rare adverse effect of topiramate is oligohidrosis and hyperthermia. Patients, especially children should be monitored for decreased sweating and hyperthermia. Use caution when prescribing drugs that predispose patients to heat-related disorders (anticholinergic drugs and carbonic anhydrase inhibitors). Must be gradually discontinued to avoid status epilepticus. Pregnancy Category D and has been found to be teratogenic in animal studies. Excreted in breast milk. Tiagabine: Patients without epilepsy who are prescribed tiagabine may have seizure or status epilepticus. Seizures have been reported in doses as low as 4mg/day. Most of these patients were taking other medications concomitantly such as antidepressants, antipsychotics, stimulants, or narcotics. Should not be abruptly discontinued because it may lead to increased seizure activity. Increased risk of suicidal ideation. Pregnancy Category C. Tetratogenic in fetal rats. Excreted in breast milk and not recommended in lactating women unless there are no other options for maternal treatment. Adverse Drug Reactions: Gabapentin: Most common CNS adverse effects of gabapentin include somnolence and dizziness. Less common CNS effects include ataxia and abnormal thinking. Peripheral edema may occur as well. Topiramate: Ataxia, parathesia, dizziness, somnolence, and difficulty concentrating. Anorexia, difficulty with memory, weight loss confusion, depression, mood problems, and psychomotor slowing. Increase in incident of kidney stones. Hyperammonemia with or without encephalopathy has been observed in clinical trials in patients taking topiramate both with and without concomitant valproic acid use. Metabolic acidosis. Tiagabine: Difficulty concentration, dizziness, light-headedness, somnolence, confusion, asthenia or lack of energy, and nervousness or irritability. Suicidal thoughts. Drug Interactions: Gabapentin does not interfere with the metabolism of commonly coadministered AEDs. Coadministration of naproxen increases absorption of gabapentin 12% to 15%. Gabapentin may increase the effects of alcohol and other CNS depressant drugs. Gabapentin may cause a false-positive urinary protein level with the N-Multistik SG test. Monitoring: Seizure frequency, duration, and severity are monitored with all AEDs, as should mood changes, signs of depression, anxiety, and suicidal thoughts. Patients taking gabapentin, topiramate, and tiagabine do not require routine serum drug levels. Patients taking topiramate should have serum electrolyte, including sodium bicarbonate, monitored at baseline and periodically. Weight is monitored in all patients on topiramate as are body temperature and the ability to sweat. Serum ammonia levels should be drawn in any patient taking topiramate who exhibits any change in level of consciousness, unexplained lethargy, or vomiting. Patient Education: Missing doses may cause an increase in seizures. Patients should be warned about the possibility of suicidal thoughts, behavioral changes, somnolence, dizziness, or balance issues. Any signs of confusion, unexplained vomiting, or mental status change in patients taking topiramate require investigation for elevated ammonia levels or metabolic acidosis. Levetiracetam (p. 241): Antiepileptic indicated as an adjunct drug in the treatment of partial onset seizures in children and adults. Own unique drug class because it is chemically unrelated to other AEDs. Pharmacodynamics: It does not appear to inhibit or affect GABA, nor does it affect calcium or sodium currents or channels. Clinical trials have shown that levetiracetam inhibits burst firing without affecting normal neuronal excitability, suggesting that levetiracetam may prevent epileptiform burst firing and spread of seizure activity. Precautions and Contraindications: Increased risk of suicidal thoughts, depression, and changes in mood and behavior. Can also cause somnolence, fatigue, dizziness, and muscle coordination difficulties. Potential for withdrawal seizures if levetiracetam is abruptly stopped. May cause transient decrease in WBC count. Pregnancy Category C. Altered pharmacokinetics during pregnancy may affect Keppra serum concentrations; with decreased serum concentration reported during pregnancy. Approved for use in children age 1 month or older as adjunct therapy in partial seizures and as adjunctive therapy in children 6 years and older with primary generalized tonic-clonic seizures. Adverse Drug Reactions: Somnolence, dizziness, behavioral changes (nervousness, anxiety, hostility, and emotional lability), alopecia, suicidal thoughts. Drug Interactions: Mostly unbound and does not use CYP450 enzymes for metabolism, thereby decreasing the likelihood of drug interactions. Does not interact with phenytoin, valproate, carbamazepine, gabapentin, lamotrigene, or phenobarbital. Does not interact with oral contraceptives or warfarin. Monitoring: Routine lab monitoring of patients on Keppra is not necessary. Efficacy is determined by decrease in the mean weekly frequency of partial onset seizures. Patient Education: Withdrawal seizures may occur if med is abruptly discontinued. Patient should be warned about potential for suicidal thoughts and actions, behavioral changes, somnolence, dizziness, or balance issues. Lamotrigine (p. 243): Used as adjunctive therapy in adults and children 2 or older in the treatment of partial seizures, primary generalized tonic-clonic seizures, and generalized seizures associated with Lennox-Gastaut syndrome. Approved for maintenance treatment of bipolar I disorder to delay occurrence of mood episodes in adults age 18 or older. Pharmacodynamics: It is thought that lamotrigine affects voltage-sensitive sodium channels and inhibits presynaptic release of glutamate and aspartate in the neuron. Precautions and Contraindications: Life threatening hypersensitivity reactions have occurred; these include multiorgan failure or dysfunction, hepatic abnormalities, and disseminated intravascular coagulation. Early symptoms of hypersensitivity, such as fever or lymphadenopathy, require evaluation and discontinuation if the reason for symptoms cannot be established. Blood dyscrasias, including neutropenia, leukopenia, anemia, thrombocytopenia, pancytopenia, and aplastic anemia have been reported in patients taking lamotrigine. Black-Box Warning regarding Stevens-Johnson Syndrome, toxic epidermal necrolysis, and rash-related death. Coadministration with valproate may increase the risk of rash. Increased risk of suicidal thoughts, depression, and mood changes. Lamotrigine should be withdrawn slowly over at least 2 weeks to prevent seizures. Pregnancy Category C, excreted in breast milk. Has low protein binding and maternal plasma levels may rise postpartum leading to elevated milk levels. Adverse Drug Reactions: Black Box Warning for life-threatening rashes such as Stevens-Johnson Syndrome. Multiorgan failure has been reported. Blood dyscrasias may occur. Increased suicidal thoughts or behavior. Abruptly stopping the med can cause withdrawal seizures. Drug Interactions: The enzyme inducers carbamazepine, phenytoin, phenobarbital, and primidone decrease lamotrigine concentrations by approximately 40%. Oral estrogen- containing contraceptives decrease lamotrigine levels by about 50%. If the patient is already taking lamotrigine and an oral contraceptive is started, the lamotrigine dose may need to be twice the normal dose. Rifampin decreases lamotrigine concentrations by 40% when taken concurrently. The most concerning interaction with lamotrigine is with valproate. When valproate is administered concurrently with lamotrigine there is an increased risk of SJS. Valproate also increases lamotrigine levels by more than 2-fold. Monitoring: Patients should be monitored closely for any newly occurring rash and for hypersensitivity reactions. CBC and differential should be monitored as well as renal and hepatic function. Watch for s/s of suicidal thoughts or mood changes. Patient Education: Tablets should not be crushed or chewed. Any rash needs to be reported to the provider immediately. Should be warned about possibility of suicidal thoughts and action and behavioral changes. TCAs (p.245): (amitriptyline, nortiptyline, imipramine, doxepin, trimipramine maleate, amoxapine, desipramine, protriptyline HCL, and clomipramine). TCAs cost less than other antidepressants, but have much more troublesome side effects. TCAs are less safe in treating depression in those who are at high risk for suicide, because overdose can be fata, whereas the newer antidepressants are much less likely to be fatal. Pharmacodynamics: Act on neurotransmitters serotonin and norepinephrine by inhibiting their reuptake at the presynaptic neuron. However, they also act on histamine (contributing to drowsiness and weight gain) and acetylcholine. Loxapine, an active metabolite of amoxapine, acts as an antipsychotic by blocking the dopamine receptor. Precautions and Contraindications: Due to their direct alpha-adrenergic blocking effect and quinidine-like effect on the myocardium, TCAs are contraindicated with cardiovascular disorders. • Due to their acetylcholine blocking effect they should be used with caution in those who have glaucoma, prostatic hypertrophy, or urinary incontinence. • They should not be prescribed with MAOIs or to individuals who have demonstrated hypersensitivity in this class. • Pregnancy Category C. • Tardive dyskinesia and neuroleptic malignant syndrome have been reported and are more likely with amoxapine use due to its dopaminergic effect. • TCAs should be titrated gradually. • Nausea, headache, vertigo, malaise, and nightmares may occur after abrupt discontinuation of the drug. • Most significant risks related to TCA use are cardiac conduction disorder. Children and elderly at highest risk; therefore, baseline ECG and periodic monitoring should be performed. • Most common cardiovascular effect is sinus tachycardia due to the inhibition of norepinephrine reuptake and anticholinergic action. • TCAs contribute to slowing of depolarization of the cardiac muscle, contributing to prolongation of the QRS complex and the PR/QT intervals. • The index between therapeutic and toxic levels is narrow. Great care needs to be taken when prescribing for a person who is depressed and has suicidal ideas. Such patients need to be monitored on a weekly basis. • Should be used with extreme caution if at all with the elderly. Due to their anticholinergic and norepinephrine effects, they can contribute to confusion, orthostatic hypotension, and falls. Adverse Drug Reactions: Anticholinergic adverse effects are common and can include dry mouth, constipation, urinary hesitancy or retention, blurred vision, sedation, orthostatic hypotension, weight gain, nausea and vomiting, gynecomastia, and changes in libido. Patients newly prescribed a TCA should be cautioned about safety in a situation in which mental alertness is required. Drug Interactions: The most significant drug interactions are those that increase the plasma level of the TCA and thereby increase the risk of cardiotoxicity, such as concurrent use of SRIs, cannabis, and sympathomimetics. Hyperprexia can occur with MAOIs and TCAs. Clinical Use: Efficacy in depression, panic disorder, enuresis, and chronic neuropathic pain. Due to their serotonergic and noradrenergic effects, they are especially helpful with anxiety disorders such as OCD (clomipramine) and panic disorder (imipramine). TCAs used to treat insomnia include doxepin, amitriptyline, and trazodone. Amitriptyline and imipramine are useful for neuropathic pain. MAOIs (p. 248): Infrequently used because safer drugs are available. Primarily reserved for the treatment of refractory unipolar depression. (phenelzine-Nardil, isocarboxazid- Marplan, tranylcypromine-Parnate, and selegiline-Emsam). Pharmacodynamics: Irreversibly inactive the enzymes that metabolize norepinephrine, serotonin, and dopamine, thereby increasing the bioavailability of these neurotransmitters. Prevent the breakdown of tyramine, found in many foods that are aged or fermented. Because tyramine is toxic to humans, contributing to rapid extreme HTN, these drugs require careful dietary restrictions. Selegiline transdermal (Emsam) requires dietary restriction only for doses above 9mg in 24 hours. Precautions and Contraindications: Contraindications include liver or kidney disease, hypersensitivity, congestive heart failure or arteriosclerotic disease, and age over 60 years. They should not be used in patients who are impulsive, cognitively impaired, or cannot follow the necessary dietary restrictions. Pregnancy Category C. Excreted in breast milk. Not approved for use with children. Postural hypotension and suppression of myocardial pain may occur. Adverse Drug Reactions: Initial adverse effects may include insomnia, anxiety, and agitation as a result of the delayed metabolism of dopamine. In addition, dry mouth, blurred vision, urinary retention, and constipation occur because of anticholinergic activity. Most common side effects include dizziness, headache, insomnia, restlessness, and hypotension. Clinical Use: Because safer and more convenient drugs are available, the MAOIs are reserved for drug-resistant, refractory depressions. Drug and Food Interactions: Because MAOIs inhibit the metabolism of norepinephrine, HTN crisis can occur if they are administered concurrently with other drugs or foods that raise BP, including anticholinergics, sympathomimetics, stimulants, and foods containing tyramine. Tyramine is a precursor to dopamine, norepinephrine, and epinephrine. Foods that have been aged or fermented are rich in tyramine; therefore, dietary restriction apply during use or within 14 days following discontinuance of the MAOI. • Symptoms of HTN crisis include headache, heart palpitations, stiff or sore neck, chest tightness, tachycardia, sweating, and dilated pupils. Patient should remain standing until crisis is managed. Usual treatment is phentolamine 5mg IV and then 0.25-0.5mg IM every 4-6 hours. • The prolonged metabolism of norepinephrine and the pressor effect of other drugs can lead to interactions resulting in hypotension and HF. • The 14 day restriction also applies to initiating SRI or SNRI drug treatment. The increased amount of serotonin available due to inhibition of its metabolism by the MAOI leads to a risk of serotonin syndrome. As a result of other drug interactions, particularly with meperidine, CNS depression can also occur. • Avoid foods including cheese, yogurt, sour cream, aged meat and meat products, dried fish and herring, alcohol, fermented vegetables such as sauerkraut, soy sauce, miso soup, bean curd, fava beans, avocados, bananas, raisins, caffeine, chocolate, and ginseng. Monitoring: Periodic LFTs should be performed and the drug discontinued if any abnormalities are found. SSRIS (p. 249): (fluoxetine-Prozac, paroxetine-Paxil, sertraline-Zoloft, fluvoxamine- Luvox, citalopram-Celexa, escitalopram-Lexapro, and vortioxetine-Brintellix). Pharmacodynamics: Affect the serotonin neurotransmitter in the synaptic cleft by blocking the serotonin transporter from returning remaining serotonin to the presynaptic cell. Each drug has different effects on other neurotransmitters. For example, fluoxetine affects dopamine that contributes to the development of side effects. Citalopram and escitalopram are probably the closest to a true serotonin selective reuptake inhibitor. Through this mechanism, more serotonin is available to bind with the postsynaptic receptors. Metabolism and Excretion: The SSRIs have a significant first-pass effect in the liver and are metabolized predominantly by the CYP450 system. Consideration of the half-life requires consideration of active metabolites as well as the possibility of inhibiting the SSRI’s own metabolism. For example, sertraline has a half life of 24-26 hours but inhibits the P450 2D6 enzyme that is also the substrate for metabolism.Excretion of the SSRIs is primarily by the kidneys. Precautions and Contraindications: Concurrent or within14 days of administration of MAOI. They should be used cautiously in patients with severe hepatic or renal impairment and should be avoided in the first and last trimesters of pregnancy. Pregnancy: Fluvoxamine and vortioxetine are Pregnancy Category C, paroxetine is Pregnancy Category D, and the others are Category B. High doses of SSRIs have caused malformations and fetal death in animal studies. Neonates exposed to SSRIs in the late third trimester have demonstrated complications soon after birth, requiring respiratory support and tube feedings. Children: Paroxetine is not approved for use in children after studies indicated it is not effective for them. Vortioxetine has not been studied for safety or effectiveness in children. Suicidal Thoughts: There is a greater risk of suicide within the first 3 weeks of taking SSRIs and other antidepressants. This is related to the lag time in receiving the full therapeutic effect while there is an increase in neurocognitive activation early in initiation of the drug. Adverse Drug Reactions: Most common are nausea, vomiting, headache, light- headedness, dizziness, dry mouth, increased sweating, weight gain or loss, exacerbation of anxiety, and agitation. A significant adverse effect is serotonin syndrome (nausea, diarrhea, chills, sweating, hyperthermia, HTN, myoclonic jerking, tremor, agitation, ataxia, disorientation, confusion, and delirium), which occurs in the presence of excessive serotonergic activity. Therefore, maximum recommended doses must be adhered to, adjunctive combinations of serotonergic agents must be avoided, and adequate time for titration when changing from one serotonergic to another must be provided. A safe guideline when making such a change is to allow five half lives per dose decrease, so that titrating off a 20mg dose of paroxetine would need 5 days at 10 mg before starting another serotonergic drug. Withdrawal: In shorter half life drugs such as paroxetine, sertraline, citalopram, and escitaloproam can show withdrawal symptoms with just one missed dose. These symptoms are nausea, dizziness, and paresthesias such as electric shock sensations or visual tracers with eye movements. **Fluoxetine is the only SSRI that DOES NOT require gradual and slow tapering because of its long half life and active metabolites. A single dose of fluoxetine as the last step in tapering off other SSRIs is helpful to avoid withdrawal symptoms. Drug Interactions: The most significant occur with MAOIs and other serotonergic drugs. With MAOIs there needs to be at least a 14 day washout period before initiating an SSRI and at least a 21 day washout of fluoxetine before initiating an MAOI. Drugs that inhibit the P450 2D6 will increase the effects of SRI, and many SRIs inhibit the 2D6 and 3A3/4 and will interact with drugs that use these enzymes as substrates. CNS depression can occur with alcohol, antihistamines, and opioids. St. John’s wort and/or SAMe may contribute to serotonin syndrome. SSRIs should NOT be prescribed with TCAs and require washout between drugs. The SSRI may increase the plasma level of the TCA, which increases the risk of cardiac conduction complications. Clinical Use: Citalopram: Depression, anxiety (off-label). Escitalopram: Depression, anxiety. Fluoxetine: Depression, OCD, bulimia, panic disorder. Fluvoxamine: OCD, social anxiety disorder, depression (off-label). Paroxetine: Depression, OCD, panic disorder, social phobia, PTSD, anxiety, PMDD. Sertraline: Depression, OCD, GAD, PMDD, PTSD, social anxiety disorder. Vortioxetine: Major depression Rational Drug Selection: The SRIs, except fluvoxamine, are indicated for the treatment of depressive, anxiety, and panic disorders; OCD; and bulimia. Fluvoxamine is FDA approved for the treatment of OCD, although it is likely to be as effective as the others for listed disorders. The FDA has approved the indication for premenstrual dysphoric disorder, PTSD, generalized anxiety disorder, and social phobia. Off-label uses include the treatment of anorexia, the depressive phase of bipolar disorder, chronic headaches and other types of pain, impulse control disorders, and trichotillomania. There should be at least telephone contact on a weekly basis with an agreement to notify the prescribed immediately if suicidal thoughts occur during the first 2-3 weeks of initiation. Patient Education: Drugs may take 3-4 weeks until their full therapeutic benefits become evident and that the initial adverse reactions, commonly including nausea, intermittent light-headedness, sedation, muscle restlessness, and sleep disruptions should be minor and transient. Do not miss a dose or let the prescription run out before seeking a refill because of the withdrawal syndrome. SNRIs (p. 253): (venlafaxine-Effexor, desvenlafaxine-Pristiq, duloxetine-Cymbalta, Milnacipran-Savella, and levomilnacipran-Fetzima). Pharmacodynamics: Venlafaxine and duloxetine both block the serotonin and norepinephrine transporters, thereby inhibiting the reuptake of the neurotransmitter and increasing the availability to bind with the postsynaptic receptors. At lower doses (75mg), venlafaxine predominantly affects serotonin reuptake, contributing to greater reduction of anxiety more so than reduction of depressive symptoms. Duloxetine, however, appears to be more potent and equal serotonin and norepinephrine reuptake inhibitor than venlafaxine. For milnacipran and levomilnacipran it is thought that they inhibit reuptake of serotonin and norepinephrine. Metabolism and Excretion: Venlafaxine is metabolized by CYP450 2D6 with one active metabolite and two less active metabolites. Duloxetine is metabolized by CYP450 2D6 and 1A2. Venlafaxine has a half-life of 5 hours, and the active metabolite has a half- life of 11 hours. Steady state is achieved in 3-4 days. Duloxetine has a half-life of 12 hours, reaching steady state in 3 days. Both drugs are excreted mostly in the urine. Levomilnacipran is catalyzed primarily by CYP3A4 and is excreted renally 55% of milnacipran is excreted unchanged in the urine. Milnacipran is also metabolized into inactive metabolites that are excreted in the urine. Precautions and Contraindications: Increased suicidal thinking during the first few weeks of initiation and change in dosage of the medication. The patient must be monitored at least weekly and assessed for suicide risk each time. Pregnancy: Pregnancy Category C, no well controlled studies in pregnant women taking SNRIs. There have been reports of fetal mortality and skeletal malformations in rats and rabbits. Neonates of women who took SNRIs in the 3rd trimester have developed complications soon after birth requiring respiratory support and tube feeding. Both venlafaxine and duloxetine have been found in breast milk. No proven adverse effects to infants, but it is recommended that infants be monitored. Levomilnacipran is not recommended for nursing women. Glaucoma: Duloxetine may exacerbate narrow-angle glaucoma and should be used cautiously with these patients. Liver: Duloxetine has also shown increased serum transaminase levels and should not be used with patients with liver disorders. Adverse Drug Reactions: Most common side effects of venlafaxine and duloxetine include headache, somnolence, dizziness, insomnia, nervousness, nausea, dry mouth, constipation, and abnormal ejaculations. Appetite and weight decreases may occur. At higher doses both drugs may contribute to elevated blood pressure. Milnacipran: The most common adverse effect of milnacipran in clinical trials was nausea, other symptoms include headache, constipation, hot flush, dizziness, hyperhidrosis, and palpitations. Male patients reported ED, decreased libido, and ejaculation failure. Levomilnaciprain: Nausea, constipation, hyperhidrosis, tachycardia, ED, and urinary hesitation. Drug Interactions: CYP2D6: Drugs that inhibit CYP450 2D6 will interact with both venlafaxine and duloxetine, including fluoxetine and quinidine. With duloxetine, drugs that inhibit 1A2 will also interact, especially fluvoxamine and some quinolone ABX. Frequent alcohol use may affect liver function and therefore duloxetine should not be used with patients who abuse or are dependent on alcohol. CYP3A4: Drugs that inhibit CYP3A4 (ketoconazole) may require adjustment of levomilnacipran dosage. Alcohol may cause an accelerated release of levomilnacipran ER, and it is recommended that levomilnacipran not be taken with alcohol. Serotonin Syndrome: May occur if milnacipran is used with a triptan. If a triptan is indicated, patients should be monitored closely. Catecholamines: Epinephrine and norepinephrine may cause paroxysmal HTN and dysrhythmias if coadministered with milnacipran because milnacipran inhibits the reuptake of norepinephrine. Digoxin: Patients taking milnacipran and digoxin have reported postural hypotension and tachycardia. Clonidine: Clonidine’s antihypertensive effect may be inhibited by milnacipran owing to inhabitation of norepinephrine reuptake. Clinical Use: Venlafaxine: Anxiety disorders such as generalized anxiety disorder, social phobia, and PTSD. Available in extended release form and immediate release form. The immediate release form must be taken at least BID and has uncomfortable discontinuation symptoms if doses are missed, including paresthesias, dizziness, nausea, and vomiting. The initial dose of extended release is 75mg/day, which is increased to 150-300mg/day in increments of 75mg every 4 days. Severely depressed patients may require higher dosage of 375-450mg/day in divided doses. Duloxetine: Neuropathic pain and overactive bladder. Initial dose is 20mg/day, which is increased to 60mg/day in increments of 20mg every 4 days. Levomilnacipran: Major depressive disorder, with the recommended dose range of 40mg-120mg daily. Patients should be started on 20mg once daily for 2 days, then increased to 40mg daily. The dose may be adjusted in increments of 40mg every 2 days until efficacy is determined. Max dose 120mg/day. Withdrawal symptoms may be seen if discontinued abruptly; should be tapered off. Milnacipran: Fibromyalgia with recommended dose of 100mg/day (50mg BID). Withdrawal symptoms may be seen if stopped abruptly; should be tapered off. Patients may tolerate better if dose is titrated based on the following schedule: Day 1: 12.5mg once Days 2-3: 25mg/day (12.5mg BID) Days 4-7: 50mg/day (25mg BID) After Day 7: 100mg/day (50mg BID). Rationale Drug Selection: Both venlafaxine and dulocetine are more activating than the SSRIs and therefore are first-line drugs to use with patients who have the more sluggish types of depression. Venlafaxine also seems effective with adults who have both depression and ADHD. Milnacipran is only used for fibromyalgia. Monitoring: No specific serum level monitoring is available for the SNRIs. With duloxetine, liver function should be monitored once weekly, once monthly, biannually, and finally annually. Monitor for suicidal risk. Patient Education: Patients should be given written descriptions of side effects and ways to relieve them. Women who are pregnant need to be tapered off medication, especially in the third trimester. Sudden discontinuation usually leads to uncomfortable withdrawal symptoms; and patients need to request refill prescriptions in an adequate amount of time to avoid running out. Drug interactions: MAOIS. Table 15-13 (p. 249) Drug Interacting Drug Possible Effect Implications All MAOIs Anorexiants, venlafaxine, SSRIs, bupropion, bromocriptine, L- dopa, L-tryptophan, MAO-B inhibitor, sumatriptan Increased serotonergic effect, possible serotonin syndrome Avoid CNS depressants, meperidine, antipsychotics Increased CNS depression Use cautiously in hazardous situations Amphetamines Buspirone, L-dopa, reserpine, tetrabenazine, guanethidine, meperidine Increased BP and possible hypertensive crisis Monitor BP; avoid if possible Antihypertensives, propoxyphene, meperidine, diuretics, nitroglycerin, dextromethorphan Hypotension agitation diaphoresis, vascular collapse Monitor BP; avoid concurrent administration if possible Insulin, sulfonylureas Hypoglycemia Monitor blood glucose and for s/s of hypoglycemia Carbamazepine Increased carbamazepine level Monitor level; use alternative anticonvulsant if possible TCAs and SSRIs Seizures and delirium Avoid Typical Antipsychotics (p. 256): The phenothiazine group of typical APs includes chlorpromazine (Thorazine), thioridazine (Mellaril), fluphenazine (Prolixin), and fluphenazine decanoate, pephenazine (Trilafon), and trifluoperazine (Stelazine). Nonphenothiazine typical APs include haloperidol (Haldol) and haloperidol decanoate, thiothixene (Narvane), loxapine (Loxitane), and molindone (Moban). Pharmacodynamics: The typical APs block D2 receptors in the basal ganglia, hypothalamus, limbic system, brainstem, and medulla and reduce the positive symptoms of schizophrenia. Less effective in treating the negative symptoms of schizophrenia, such as flat affect, decreased motivation, withdrawal from interpersonal relationships, and poor grooming and hygiene. Clinical effectiveness occurs when 60-70% of D2 receptors are blocked. Too much dopamine blockade, however, leads to symptoms resembling those of parkinsonism. As D2 occupancy nears 78%, extrapyramidal symptoms (EPSs) are more prominent. Precautions and Contraindications: High potency drugs such as haloperidol and fluphenazine carry an increased risk of causing EPSs, whereas low potency drugs such as chlorpromazine and thioridazine carry less risk of EPSs but more risk of anticholinergic adverse reactions (dry mouth, constipation, urinary retention, blurred vision) and antiadrenergic effects (orthostatic hypotension). Contraindications include narrow-angle glaucoma, bone marrow depression, and severe liver or cardiovascular disease. These agents should be used cautiously in the presence of CNS tumors, epilepsy, DM, respiratory disease, and prostatic hypertrophy. Safety is not established in pregnancy and lactation. All antipsychotic medications have a Black-Box Warning regarding increased mortality in elderly patients with dementia-related psychosis and are not approved for the treatment of such patients. ADRs: A life threatening ADR is neuroleptic malignant syndrome (NMS), characterized by fever up to 107 degrees F, elevated pulse, diaphoresis, rigidity, stupor or coma, and acute renal failure. EPSs are among the most troublesome side effects and include pseudo parkinsonism (shuffling, pill rolling, cog wheeling, tremors, drooling, rigidity), akathisia (restlessness), dystonia (involuntary, painful movements), and tardive dyskinesia (involuntary buccolingual movements and difficulty speaking and swallowing). Antiparkinson, antihistamine, and anticholinergic drugs are given to counter EPSs. Other side effects include sedation, weight gain, anticholinergic effects, photosensitivity, reduction of seizure threshold, orthostatic hypotension, sexual dysfunction, galactorrhea (milky nipple discharge), and amenorrhea. Clinical Use: Typical APs are more effective in reducing the positive than they are the negative symptoms of schizophrenia. May be more effective than atypical APs in treating very severe psychosis. Patients who need rapid control of agitation and dangerous psychosis can be treated with IV haloperidol. IM chlorpromazine also provides rapid sedation. Rational Drug Selection: The choice of a specific agent can be guided by past response to the medication, initial response, family history, and side effect profile of the medication. Usually EPSs can be decreased by the addition of drugs such as benztropine (Cogentin), diphenhydramine (Benadryl), trihexyphenidyl (Artane), and atenolol (Tenormin), or amantadine (Symmetrel). A decrease in the dose or a change to a different type of antipsychotic may also counter these effects. Some anticholinergic effects such as constipation can be addressed by nonpharmacological measures such as increased fluid intake and dietary bulk. The depot (injection, slow release, slow acting) or decanoate form of medication may be used if compliance is an issue. It is usually administered every 2-4 weeks. Monitoring: The motor function of individuals taking typical antipsychotics should be routinely assessed with the Abnormal Involuntary Movement Scale (AIMS), which rates various movements such as joint rigidity and balance numerically, thereby enabling the clinician, over time, to detect changes that represent early EPSs. May elevate prolactin levels because dopamine, which inhibits prolactin, is blocked. Patients should be monitored for the consequences of chronic prolactin elevation such as galactorrhea, gynecomastia, amenorrhea, and sexual dysfunction. Atypical Antipsychotics (p. 259): arpiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal), and ziprasidone (Geodon). Address both the positive and negative symptoms of schizophrenia. Some of the superioirity in treating negative symptoms, as compared to typical APs may be related to less interference with cognitive functioning. Because there is a better patient tolerability than with typical APs, patients are more likely to continue taking the atypical APs. These newer agents are characterized by less risk of EPSs, TD, and elevation of prolactin levels. The APs are associated with unhealthy weight gain, which leads to a metabolic syndrome (abdominal obesity, high BP, high cholesterol levels, and insulin resistance). Schizophrenia itself, as well as atypical APs increases the risk of diabetes. Before starting any atypical AP, patients should be assessed for waist circumference, BMI, BP, fasting plasma glucose, and lipid profile. The practitioner should recheck BMI monthly and order laboratory work-ups at 3 months. After 3 months, BMI should be checked quarterly and BP, lab workups and waist circumference annually. Pharmacodynamics: Thought to block serotonin receptors in the cortex, which blocks the usual ability of serotonin to inhibit the release of dopamine. Thus, more dopamine is released to the prefrontal cortex, which reduces the negative symptoms of schizophrenia. All drugs with antipsychotic properties block dopamine D2 receptors, but atypical APs generally have less D2 blockade than the typical APs. Drugs with the least D2 blockade (clozapine, olanzapine) have the lowest incidence of EPSs. Most of the atypical APs also variously affect adrenergic, histaminic, and cholinergic receptors. Drugs that are potent histamine H1 receptor antagonists (olanzapine, clozapine) produce more weight gain and sedation. Drugs that block noradrenergic receptors (clozapine) produce more hypotension. Precautions and Contraindications: Most atypical APs are not recommended in pregnancy (Pregnancy Category C), lactating women, or young children. Lurisidone is Category B. APs should be prescribed cautiously in the presence of hepatic or renal disease. Because of liver function decline, the geriatric population generally requires smaller doses. ADRs: Less risk of EPSs, TD, NMS than with typical APs. Significant negative adverse effects include seizures, weight gain, diabetes, hyperprolactinemia, dizziness, orthostatic hypotension, tachycardia, sleep disturbance, constipation, and rhinitis. Clozapine: Risk of potentially fatal agranulocytosis (potentially lethal in 24-72 hours and requires immediate attention). Reserved for the treatment of severe schizophrenia refractory to complete trials of at least two different types of APs. Clozapine is available only through a patient management system in which a clinician and patient are both registered. A baseline CBC with differential is obtained prior to treatment, then monitored weekly or biweekly, depending on the length of time the patient has been taking clozapine, before the next week’s medication is dispensed by the pharmacy. Monitoring should be continued for 4 weeks after clozapine is d/c’d. The clinician must be aware of the indications of a falling WBC (fever, lethargy, bruising, sore throat, flu-like sxs). Aripiprazole: Relatively weight neutral and lacks any significant effect on QT intervals. It has god antidepressant properties but may be unpleasantly activating to some patients. Side effects include agitation, akathisia (muscle quivering, restlessness), nausea, tremor, insomnia, and headache. Asenapine: May cause somnolence and dose-related akathesia. Patients taking 10mg daily of asenapine for bipolar disorder may report oral hypoesthesia (numbness). Iloperidone: Tachycardia, nausea, dry mouth, dizziness, somnolence, and orthostatic hypotension. Risperidone: Dose should be titrated up slowly over a few days or longer to minimize adverse effects. Orthostatic hypotension, bradykinesia, akathisia, agitation, and elevation of prolactin levels. Weight gain with risperidone is generally less than it is with clozapine or olanzapine. Olanzapine: Long term use leads to sedation and weight gain. This weight gain appears to be associated with increased appetite, with much of the weight gain occurring in the first 6 months of drug therapy. Should be taken at bedtime. Low incidence of EPSs. Paliperidone: Tachycardia, somnolence, akathesia, and EPSs. Quetiapine: Dizziness and somnolence. Weight gain and orthostatic hypotension may also occur. Ziprasidone: Generally well tolerated. May cause weight loss and reduced triglyceride levels. Low incidence of EPSs. Most common side effects are drowsiness, dyspepsia, dizziness, constipation, and nausea. One concern is that ziprasidone is associated with mild-moderate QT interval prolongation. Patients with known history of arrhythmia should have a baseline and repeat ECG. . Clinical Use: Table 15-22 (p. 262). Rational Drug Selection: Indications for use include schizophrenia, schizoaffective disorder, depression or mania with psychotic features, and severe agitation and delusions with dementia. Selecting one atypical antipsychotic over another may be based on specific patient risk factors, history of response to specific medications, or adverse effects experienced by the patient. Change from one AP to another should be accomplished by slowly titrating off the first medication and onto the second, with a washout period in between if possible. If presence of psychotic symptoms makes a washout period unfeasible, overlap of medications should be at the lowest doses and for the shortest period of time possible. Monitoring: No specific blood tests are available to determine the plasma level of these medications. Dosages are adjusted based on subjective information provided by the patient and the clinician’s objective observations of the client. Atypical Antipsychotics Used in Treatment of Major Depressive Disorder: Quetiapine (Seroquel) can be used adjunct to major depressive disorder antidepressant treatment. 50mg/day at HS, then increase by 100mg qHS until 300mg on day 4. May increase to 400mg day 5 and 600mg day 8 if needed. Dopaminergics (p. 264): (AKA dopamine agonists). Treatment of choice for Parkinson’s disease. These agents include amantadine (Symmetrel), bromocriptine (Parlodel), carbidopa-levodopa (Sinemet), cabidopa-levodopa-entacarpone (Stalevo), selegiline hydrochloride (Eldepryl), pramipexole (Mirapex), rasagiline (Azilect), ropinirole (Requip), and tolcapone (Tasmar). Amantadine is occasionally used to treat the parkinsonism-like EPS of the antipsychotic drugs, but to give a dopamine-enhancing drug to a patient with schizophrenia might cause psychotic symptoms to increase. Pharmacodynamics: Dopamine and acetylcholine are the neurotransmitters primarily responsible for balance and coordinated musculoskeletal functioning, and each needs to balance the other for smooth functioning to take place. When dopamine depletion occurs, either idiophatically as in Parkinson’s’ or because of inadequate synthesis or impaired storage, transmission, or reuptake, the classic signs of muscular rigidity, tremors, and psychomotor retardation appear. Excessive amounts of dopamine are thought to produce the positive symptoms of schizophrenia, such as hallucinations and delusions. Amantadine: Effective because it releases dopamine from storage, whereas the dopamine precursors levodopa and carbidopa-levodopa increase dopamine synthesis. Entacapone: When given with levodopa and carbidopa, the plasma levels of levodopa increase and are higher and more sustained when given with just carbidopa. Bromocriptine and Pergolide: Act as dopamine agonists at the postsynaptic receptor sites. Selegiline: Inactivates MAO, which then leads to increased amounts of dopamine available in the CNS. Rasagiline: Acts as a selective, irreversible MAO inhibitor. Pramipexole and Ropinirole: Act by stimulating dopamine receptors in the brain. Tolcapone: Is thought to enhance the pharmacokinetics of levodopa. Precautions: Should be used cautiously in patients with a history of cardiac, psychiatric, or ulcer disease. Pregnancy Categories B and C; their safety during lactation and in children has not been determined. Selegiline: Contraindicated with concurrent administration of meperidine. Amantadine: Renal impairment should be carefully assessed before using because it is excreted unchanged through the kidneys. Pergolide: Patients with underlying cardiac arrhythmias who have taken Pergolide have experienced bradycardia and sinus tachycardia. Ropinirole and Pramipexole: Should be used cautiously in geriatric patients because of the increased risk of hallucinations. Carbidopa-levodopa: Contraindicated in narrow-angle glaucoma and malignant melanoma. Tolcarpone: Contraindicated in patients with liver disease. ADRs: Nausea, vomiting, dizziness, postural hypotension, abdominal pain, dyspepsia, constipation, dry mouth, depression, insomnia, confusion, and hallucinations. Pramipexole and Ropinirole: May cause sleep attacks in which the patient has unexpected episodes of falling asleep. Tolcapone: May cause severe hepatocellular injury, including liver failure. Anxiolytics and Hypnotics (p. 267): Drugs used to treat anxiety can be divided into three groups: serotonergics, gabaergics, and dopaminergics. Traditionally, the benzodiazepines (diazepam, alprazolam) were regarded as anxiolytics. The benzodiazepines affect the GABA receptors at a particular site within the receptor, whereas other GABA-ergics affect the receptor more globally. The net effect of inhibiting GABA is to slow down neurotransmission and thereby produce reduction in anxiety. Serotonin as a neurotransmitter has a calming effect as well, owing to the areas of the brain where there are high concentrations of these pathways. Finally, dopaminergics have an anxiolytic effects similar to that of serotonin but in more specific areas of the brain. Therefore, the prescriber needs to select a drug based not only on the general class of drugs but also on the specific symptomatology produced by the neurophysiology. Benzodiazepines: Because of the increased potential for tolerance and dependence, the CNS depressant-related adverse effects, and the development of buspirone, many clinicians are more cautious in assessing risks versus benefits for their patients than they might have been previously. Alprazolam (Xanax), chlordiazepoxide (Librium), clonazepam (Klonopin), clorazepate (Tranxene), diazepam (Valium), halazepam (Paxipam), lorazepam (Ativan), oxazepam (Serax). Can be used to treat anxiety, muscle relaxant, preanesthesia sedation, prevention and treatment of panic attacks, acute agitation and dystonia, emergency treatment for uncontrollable seizures, and treatment of restless leg syndrome. Pharmacodynamics: Thought to exert their anxiolytic and sedative effects by increasing the action of GABA, an inhibitory neurotransmitter, thereby decreasing the effect of neuronal excitation. Within the GABA receptor is an area that the benzodiazepines bind to, referred to as the benzodiazepine receptor. Precautions and Contraindications: The development of dependence is of concern with benzos. Although dependence is usually related to high dose and duration of use (More than a few weeks), it can occur in the absence of these parameters. It is thought that alprazolam (Xanax) and lorazepam (Ativan) are more likely to cause dependence because of their high potency and rapid, short-term action but clonazepam (Klonopin) is less likely because of its long action. Symptoms of withdrawal, which usually occur 1-2 days after the last dose of short-acting benzos and 5-10 days after the last dose of long-acting compounds, resemble withdrawal symptoms of other CNS depressants. Use of the drug should be tapered because of the risk of severe withdrawal symptoms. One strategy for tapering is to decrease the dose by 0.5mg/week, and then by 0.25mg/week for the last few weeks. Another is to substitute a long-acting benzo such as clonazepam in an equivalent dose for a short-acting one and then titrate down. Contraindications: Pregnancy and lactation. Hepatic and renal disease. Children under 6. Acute narrow-angle glaucoma. Geriatric patients generally should not be prescribed benzos; if they are prescribed, they should be in very low doses due to their decreased rate of metabolism and consequent potential for accumulation. ADRs: Major adverse effects are due to the drugs’ action as CNS depressants. The same concerns apply to their use with other CNS depressants: excessive sedation and potential for cardiac and respiratory depression. Paradoxical anxiety, agitation, and acute rage may occur with benzos. Clonazepam may increase salivation. Other side effects include dizziness, confusion, blurred vision, and hypotension. Rational Drug Selection: Diazepam is the treatment of choice for status epilepticus, administered by parenteral route, preferably IV, because of the rapidity of absorption and effect. In acute alcohol withdrawal, care must be exercised so that cross-tolerance dose not develop. Because dependence has occurred after as little as 4-6 weeks of use, these drugs should not be used beyond the acute stage of alcohol withdrawal and should be slowly tapered. All of the benzos are equally efficacious and drug selection depends on patient and prescriber preference and the patient’s side-effect profile. For long-term treatment of anxiety, other classes of drugs should be considered first (buspirone or SSRIs); and if benzos are necessary, then clonazepam is preferred due to its long half-life and daily dosing ability. Patient Education: Avoid alcohol. Avoid taking before situations in which mental or physical alertness is required to maintain safety. Report ocular pain or changes in vision immediately. . Serotonergic Anxiolytics (p. 269): Neurophysiologically, it makes sense that enhancing serotonin would contribute to relief of anxiety because of the areas of the brain that are heavily innervated by serotonin. However, there are 15 subtypes of serotonin receptors, some of which may actually contribute to anxiety. Buspirone is a serotonergic that is a member of the azapirones, a new group of anxiolytics. These drugs exert their effects without CNS depression and sedation of barbiturates and benzos but also without the anticonvulsant or muscle-relaxant qualities. Buspirone has little risk of dependence and few drug interactions. Pharmacodynamics: Similar chemical structure to butyrophenone APs such as haloperidol. However, further human studies showed that it had greater efficacy as an anxiolytic, through its action on the serotonin-1A (5-HT 1a) presynaptic and postsynaptic receptors. At the presynaptic 5-HT 1a receptor, buspirone is a full agonist, that is, it contributes to the channel’s opening and permitting serotonin binding, thereby inhibiting neuron firing. Buspirone also is a partial agonist at the postsynaptic 5-HT 1a receptors. When there is an excess of serotonin, buspirone acts as an antagonist, but in a deficit state such as is presumed in anxiety and depression, it acts as an agonist. Inhibits the increase in dopamine D2 receptors. However, the dopaminergic action is minor compared to the serotonergic effects. Buspirone has no effect on the GABA receptor and cannot be used as a substitute for benzos in withdrawal treatment. Precautions and Contraindications: Contraindicated in severe hepatic or renal disease and treatment of panic disorder both because of its prolonged onset and possibility of exacerbating panic. Pregnancy Category B. Use during lactation should be avoided. Not commonly though to be sedating, but drowsiness should be assessed prior to use in situation requiring cognitive or motor alertness. ADRs: Most common are light-headedness, headache, insomnia, nausea, nervousness, and dry mouth. Akathisia and involuntary movements are possible although rare. Rational Drug Selection: Can be used as the sole pharmacotherapeutic modality for anxiety. Can also be used adjunctively with SSRIs in treatment-resistant depression because of the combined serotonergic mechanisms, that is, postsynaptic reuptake inhibition and receptor agonism. Buspirone is indicated in treating generalized anxiety disorder, depression with an overlay of anxiety, and situation anxieties that are long- lasting. It is essential that the drug be taken daily; it cannot be used on a PRN basis. A positive response may begin within 7-10days of starting the drug, but maximum benefits may not become evident for 3-6 weeks. It may be necessary to add a benzo in very low doses initially to relieve the patient’s anxiety. Patient Education: Advise the patient to try the medication and observe the effects, especially drowsiness, before engaging in activities requiring mental or physical alertness. The patient also needs to be told of the prolonged onset and be offered nonpharmacological strategies for anxiety management during this time. Barbituates (p. 271): Have been used historically as anxiolytics, sedative-hypnotics, and anticonvulsants. Because of tolerance and dependence problems associated with their use, the indications for short-acting barbiturates are limited to preanesthesia sedation, short- term treatment of insomnia, and uncomfortable seizure activity, such as status epilepticus. Long-acting phenobarbital (Solfoton, Mebaral) is the drug of choice for some types of epilepsy. Phenobarbital: Onset 30 minutes or more. Duration 10-16 hours. Half-life 66-140 hours. Phenobarbital IV: Onset less than 5 minutes. Peak 15 minutes or more. ADRs: Due to CNS depressant effects of the drugs and can consist of persistent sedation and drowsiness, leading to safety concerns. Respiratory depression and cardiac depression are dose related, but are still a concern especially in combination with other CNS depressants. Rebound status epilepticus may follow abrupt withdrawal of barbiturates during daily administration for treatment of seizure disorders. Monitoring: The difference between therapeutic and toxic plasma levels is not wide, and levels should be monitored frequently. The therapeutic range is 15-40mcg/mL. It is necessary to closely monitor blood levels when prescribing barbiturates with other drugs metabolized by CYP450 2D19. Phenobarbital: Schedule IV and may be included on a state NP formulary. Effective in treatment of some types of epilepsy- primarily tonic-clonic, simple partial, and complex partial seizures- because the reduction of response to stimuli raises the threshold for seizure activity. Other indications for use include preanesthetic sedation and short-term treatment of insomnia. The latter indication is a last resort because of the risk of dependence and the comorbidity of sleep disturbance and depression, raising the risk for suicide. Other than parenteral administration of phenobarbital in medical emergencies such as eclampsia and status epilepticus, barbiturates are generally given PO. Indications: Sedation, hypnotic, preanesthetic, treatment of partial and generalized tonic-clonic and cortical focal seizures, status epilepticus. Dosage: Epilepsy: Adults 50-100mg/day. Children 3-6mg/kg/day. Acute Convulsions: Children 200-320mg IM/IV, repeat q6h PRN. Children 4-6mg/kg/day IM/IV for 7-10 days to blood level of 10- 15mcg/mL. Sedation: Adults 30-120mg/day in divided doses; not to exceed 400mg/day. Children 8-32mg. Hypnotic: Adult 100-200mg. Children dose based on age and weight. Pre-operative Sedation: Adults 100-200mg IV 60-90 minutes before surgery. Children 1-3mg/kg IM or IV. Status Epilepticus: 15-20mg/kg IV over 10-15 minutes. . Nonbenzodiazepine Hypnotics (p. 274): Pharmacodynamics: There are three categories of nonbenzodiazepine hypnotics used to induce sleep. Act at the GABA receptor but not at the benzo site. Zolpidem (Ambien), zaleplon (Sonata), and eszopiclone (Lunesta). Ramelteon (Rozerem) is a melatonin receptor agonist with high affinity for melatonin MT1 and MT2 receptors, similar to exogenous melatonin. The first orexin receptor antagonist surorexant (Belsorma) is a neurotransmitter that regulates wakefulness, arousal, and appetite and is produced in the hypothalamus. An orexin receptor mutation is thought to cause narcolepsy. Suvorexant blocks the binding of orexin to the orexin A and orexin B receptors, promoting sleep. Precautions and Contraindications: Pregnancy Category C and should not be used in pregnancy or lactation. Use of sedative-hypnotics may lead to worsening of depression. Zolpidem, eszopiclone, and zaleplon are associated with withdrawal symptoms when discontinued. All the drugs in this class may lead to abnormal thinking and behavioral changes, including bizarre behavior and complex behaviors such as sleep driving or sleep eating. Rare cases of angioedema have been reported with remelteon. Suvorexant is contraindicated in patients with narcolepsy and should be used cautiously in patients with respiratory compromise. No sleeping medication should be used acutely beyond 3 weeks or chronically beyond 3 months without careful evaluation of the treatment plan. ADRs: The most common side effects include headache, mild transient anterograde amnesia, dizziness, somnolence, and nausea. There may be daytime drowsiness especially if taken 6 hours or less before it is necessary to awaken. Women are more sensitive to the effects of zolpidem and should be prescribed lower doses than men. Suvorexant: A dose-related worsening of suicidal ideation was reported in clinical trials of suvorexant. Mild catplexy, including periods of leg weakness lasting seconds to minutes may occur day or night. Sleep paralysis, the inability to move or speak, may occur for up to several minutes during the sleep-wake transition. Hypnagogic or hypnopompic hallucinations may occur with suvorexant. Schedule IV due to potential for abuse but does not cause physical dependence. Zolpidem, Eszopiclone, and Zaleplon: Schedule IV drugs and all exhibit withdrawal symptoms when used chronically and abruptly stopped. Ramelteon: Not a controlled substance and does not appear to cause physical dependence. Mood Stabilizers (p. 276): Used to treat bipolar disorders with evidence of depressive and manic or hypomanic episodes. Bipolar disorders are distinctive from unipolar depression by virtue of mood swings and require medication not just for depression but to restore balance in moods. This is achieved by maintaining a regularity in nerve firing as opposed to the erratic firing that produces changes in behavior and mood. The most direct way to achieve regularity is by affecting the calcium channel on the nerve axon that permits influx of ions and stimulates the release of GABA. Lithium Pharmacodynamics: The relationship between sodium, lithium, and body fluid is inverse in that when sodium and fluids are depleted, as can occur during severe vomiting, prolonged heavy sweating, and diuretic use, the level of lithium is increased. It is believed that lithium replaces sodium during depolarization in neuronal pathways, effectively stopping the transmission of electrical impulses. Precautions and Contraindications: Lithium is almost completely excreted through the renal system and it is essential that the presence of kidney disease be assessed before starting lithium. Baseline blood chemistry, including Cr, BUN, and TSH levels should be obtained. Extreme caution should be used when prescribing lithium to patients with sodium depletion or to those taking diuretics. Hypothyroidism and kidney failure may occur with long-term administration. The liver dose not metabolize lithium. ADRs: Fine tremors of the fingers, nausea, dry mouth, headache, and drowsiness. Lithium may be taken with food to minimize GI distress. Even at therapeutic blood levels, some patients may have ECG changes that are not necessarily indicative of underlying cardiac disease but should be monitored. The therapeutic range is 0.6-1.5 mEq/L. Toxicity s/s include coarse tremors of the hands that impair function, N/V/D, confusion, stupor, polydipsia, polyuria, muscle weakness, and ataxia. If the lithium level is elevated enough, coma and death can result. Treatment for overdose is supportive, including ensuring adequate hydration and even dialysis. ECG monitoring is necessary d/t lithium overdose contributing to arrhythmias. Drug Interactions: Diuretics may increase sodium excretion and increase lithium concentrations! NSAIDs and COX2 inhibitors reduce renal elimination and elevate serum lithium levels. Lithium prolongs the effects of neuromuscular-blocking agents used before surgery and during ECT. Concurrent use of etronidazole increases risk of lithium toxicity due to decreased renal excretion. Monitoring: Because s/s of toxicity may occur even at subtoxic blood levels, patients should always be assessed for tremors, nausea, and drowsiness. Lowering the dose will usually be sufficient to resolve the problems. Blood levels should be obtained 14 days after beginning treatment and 14 days after every dosage change. Routine blood levels are obtained every 3-6 months after stability is achieved. The procedure for obtaining an accurate lithium level is to have the sample drawn 12 hours after the last dose, usually the bedtime dose, before any morning dose has been taken. Routine blood counts with differential, chemistry screens, and thyroid panels should be obtained yearly. IN addition there should be baseline ECG and annual ECG to ascertain arrhythmias. Valproate (Depakote) Monitoring (p. 280): Plasma levels should be assessed to help guide dosage adjustments, with a trough concentration of 50-125mcg/mL. CBCs and chemistries should be obtained prior to onset of treatment and then every 3 months for 1 year. After 1 year, monitoring can be done annually. Non-classified Mood Stabilizers (p. 280): The nonbenzo gaba-ergics used in the treatment of epilepsy have shown effectiveness in treating bipolar states. lamotrigine (Lamictal), gabapentin (Neurontin), and topiramate (Topamax). Pharmacodynamics: All of these drugs act in some way on GABA as well as other mechanisms. Topiramate has shown the effect of hyperchloremic nonanion gap metabolic acidosis and should be used cautiously with patients with eating disorders. ADRs: Somnolence, dizziness, ataxia, fatigue, diplopia, blurred vision, nausea, and rhinitis. Drug Interactions: When given in conjunction with other antiepileptic drugs, such as carbamazepine or phenytoin, the half-life of lamotrigine is decreased, but with valproate the half-life is increased. Gabapentin reduces the bioavailability of antacids, but cimetidine increases the bioavailability of gabapentin. Gabapentin also increases the serum levels of contraceptives. Topiramate decreases the effectiveness of oral contraceptives, and carbonic anhydrase inhibitors may increase the risk of kidney stones. Monitoring: No routine serum levels necessary. Patients should monitor skin appearances and report any rashes within the first 2 months of therapy, especially if blisters form (SJS). Weight monitoring is important, especially with gabapentin. Centrally Acting Muscle Relaxants and Antispasmodics (p. 281): Commonly used centrally acting muscle relaxants include baclofen (Lioresal), carisoprodol (Soma), chloraxazone (Paraflex), cyclobenzaprine (Flexeril), methocarbamol (Robaxin), orphenadrine (Banflex), and tizanidine (Zanaflex). Pharmacodynamics:The centrally acting medications appear to act on the monosynaptic and polysynaptic spinal reflexes, rather than on the muscles themselves. Inhibiting the synaptic reflex arcs impacts the messages that are producing and maintaining the skeletal muscle spasm. Centrally acting muscle relaxants also have a sedative effect, which causes drowsiness and may enable a patient with muscle spasms to relax and sleep. Tizanidine is a centrally acting alpha2-adrenergic agonist that produces presynaptic inhibition of motor neurons in the spinal cord, reducing muscle spasticity. Precautions and Contraindications: Increase risk of falls and injury d/t CNS sedation. Many centrally acting muscle relaxants (carisoprodol, chlorzoxazone, cyclobenzaprine, methocarbamol, and orphenadrine) are on the Beer’s list of medications to be avoided in the elderly. Baclofen: Should be used cautiously in patients with decreased renal function, as it is primarily excreted in the kidneys. Patients with seizure disorder should be prescribed baclofen carefully, with seizure control and EEG monitored because of a deterioration in seizure control associated with baclofen use. Pregnancy Category C. Carisoprodol: Contraindicated in patients with porphyria or hypersensitivity to carisoprodol or its active metabolite meprobamate. May cause physical dependence and abuse or result in criminal diversion. Abuse may lead to overdose, CNS and respiratory depression, and death. Pregnancy Category C. Chlorzaxone: Rare reports of hepatotoxicity. Pregnancy Category C. Cyclobenzaprine: Contraindicated in patients with hyperthyroidism or in the acute recovery phase of an MI or who have HF, dysrhythmias, or conduction disturbances. Serotonin syndrome has been reported with use. Owing to its anticholinergic effects, cyclobenzaprine should be used cautiously in patients with urinary retention, angle-closure glaucoma, or increased IOP. Patients with hepatic impairment are not able to metabolize cyclobenzaprine well; therefore, those with mild liver dysfunction should be prescribed small doses (5mg) and those with moderate to severe liver dysfunction should not be prescribed cyclobenzaprine. Pregnancy Category B. Metaxalone: Significantly impaired renal or hepatic function. If administered to a patient with liver dysfunction, serial liver function studies should be performed. Does not have a pregnancy category. Methocarbamol: Patients with renal or hepatic dysfunction should be prescribed methocarbamol with caution. Pregnancy Category C. Orphenadrine: Significant anticholinergic effects and therefore should not be used in patients with urinary retention, prostatic hypertrophy, glaucoma, or achalasia. Pregnancy Category C. Tizanidine: Contraindicated in patients who are taking CYP1A2 inhibitors (fluvoxamine or ciprofloxacin). Anaphylaxis and angioedema has been reported with tizanidine use. Tizanidine is hepatotoxic and should be used cautiously in patients with liver dysfunction. Liver function should be monitored during use. Older adults or patients with renal dysfunction should be prescribed tizanidine cautiously due to increased adverse effects. Tizanidine is an alpha2-adrendergic agonist and patients who abruptly stop taking the medication may experience HTN or tachycardia. Pregnancy Category C. ADRs: Sedation, drowsiness, dizziness. Baclofen: Confusion, headache, insomnia, and seizures. Carisoprodol: Significant CNS and respiratory depression, which is worsened by coadministration of other CNS depressants. May cause headache, tachycardia, vertigo, atazia, syncope, insomnia, and seizures. Physical dependence may occur, as well as leukopenia or pancytopenia. Chlorzaxazone: May be hepatotoxic and may rarely cause angioedema or anaphylaxis. GI effects include N/V/D, constipation, heartburn, or GI bleeding. Cyclobenzaprine: Dry mouth, fatigue, N/V, constipation, hallucinations, and blurred vision. May cause dysrhythmias or prolonged QT syndrome. Metaxalone: Confusion, headache, anxiety, nausea, dry mouth, or GI upset. May cause leukopenia or hemolytic anemia. Methocarbamol: May cause significant sedation and dizziness. Anaphylaxis, urticaria, and rash may occur. Blurred vision, headache, leukopenia, jaundice, N/V. Orphenadrine: May cause CNS excitation, agitation, and hallucinations, dry mouth, tachycardia, syncope, palpitation, blurred vision, N/V, and abdominal cramps. Anaphylaxis may occur. Tizanidine: May cause dry mouth, asthenia (weakness), fatigue, constipation, hypotension, and bradycardia. May be hepatotoxic and liver function should be monitored. Direct Acting Antispasmotics (p. 284): dantrolene (Dantrium) and botulinum toxin type A (Botox). Pharmacodynamics: Dantrolene is administered orally or via IV and is used to treat spasticity associated with upper neuron disorders, including spinal cord injuring, stroke, CP, and MS. Botox is injected to provide localized reduction in muscle activity. Dantrolene: Causes muscle relaxation by interfering with the release of calcium from the sarcoplasmic reticulum, affecting the fast muscle fibers more than the slow fibers. The intensity of the skeletal muscle relaxation is dose-related. Botulinum Toxin Type A: Acts locally to block neuromuscular transmission by binding to receptor sites on the motor or sympathetic nerves, depending on site of injection. At the nerve terminal, botulinum toxin type A works to inhibit the release of acetylcholine. When injected intradermally, it causes temporary denervation of the sweat glands, causing decreased production of sweat locally. When injected into the detrusor muscle, botulinum toxin type A affects the afferent pathways of the detrusor muscle by inhibiting acetylcholine release. Precautions and Contraindications: Datrolene: Contraindicated in patients with active liver disease such as hepatitis or cirrhosis. May induce potentially fatal hepatocellular disease, with higher risk noted in female patients and those over age 35. The elderly may have an increased likelihood of fatal hepatotoxicity, possibly due to concomitant illness or interacting medications. Pregnancy Category C. Botulinum Toxin Type A: May spread from the site of injection to produce effects similar to botulinum toxin. Symptoms may appear hours to weeks after injection and include muscle weakness, dysphagia, dysphonia, urinary incontinence, and breathing difficulties. If muscles of the respiratory system are involved, the patient may need intubation and mechanical ventilation. If muscles of the oropharynx or esophagus are affected, patients may aspirate. Antitoxin is available for overdose from the CDC. Intradetrusor injection with botulinum toxin type A is contraindicated in patients with UTI or urinary retention. Pregnancy Category C. ADRs: Dantrolene: The major ADRs involve CNS sedation, including drowsiness, dizziness, weakness, fatigue, and malaise. These adverse effects can be reduced by starting at a low dose and slowing the increase to the target dose. Other CNS effects include mental depression, confusion, nervousness, headache, insomnia, drooling, and alteration in taste. Patients may experience diarrhea that may be severe and require the temporary cessation of therapy. If diarrhea occurs when dantrolene is restarted, the patient may not be able to tolerate the medication. Less common GI adverse effects include constipation, abdominal cramps, anorexia, nausea, and vomiting. Patients may also experience hepatitis, tachycardia, HF, urinary frequency, hematuria, ED, or urinary incontinence or retention. Hematologic adverse effects are not common but include aplastic anemia, anemia, leukopenia, and thrombocytopenia. Botulinum Toxin Type A: Adverse effects of injection are mostly related to the effects of the spread of the toxin, including dysphagia and breathing difficulties when treating cervical dystonia and bronchitis and URI in patients treated for spasticity. Patients being treated with intradetruser injections for bladder dysfunction may experience UTI, urinary retention, and residual urine volume. Patients being treated for chronic migraines reported neck pain, eyelid ptosis, muscular weakness, and musculoskeletal stiffness. Opioid Analgesics and Their Antagonists (p. 285): Opioids are classified as agonists, mixed agonist-antagonists, or partial agonists. Agonists include codeine (Tylenol #3 or #4), fentanyl (Sublimaze), hydrocodone (Vicodin), hydromorphone (Dilaudid), levorphanol (Dolophine), morphine (Roxanol), oxycodone (Percocet). Opiate antagonists include naloxone HCL (Narcan), naltrexone HCL (Revia), and nalmefene HCL (Revex). Mixed agonist-antagonists include butorphanol (Stadol), nalbuphine (Nubain), and pentazocine (Talwin). Partial agonists include nuprenorphine (Buprenex) and dezocine (Dalgan). Pharmacodynamics: Narcotic analgesics are active at various opioid receptor sites and act as agonists, partial agonists, or mixed agonist-antagonists of endogenously occurring opioid peptides. Opioids interact with mu, kappa, delta, or sigma receptors, producing both the desired and adverse effects of opioids. • The primary receptors associated with analgesia are the mu and kappa receptors. Activation of these receptors is thought to create an analgesic effect by inhibiting adenyl cyclase activity, which results in reduction in intracellular cyclic adenosine monophosphate. In addition to analgesic effect, activation of mu receptors may cause euphoria, physical dependence, and respiratory depression. Activation of kappa receptors may cause miosis, sedation, and respiratory depression. • Activation of delta and sigma receptors accounts for many of the adverse effects of opioids, such as dysphoria, hallucination, and respiratory and vasomotor stimulation. • Mixed agonist-antagonists can cause withdrawal symptoms when given to narcotic-dependent individuals because of their preference at specific opioid receptor sites. • Narcotic antagonists block or reverse opioids by competing at their receptor sites and reverse respiratory depression, hypotension, and sedation. ADRs: Opioids: Respiratory depression, hypotension, confusion, sedation, N/V, dizziness, visual disturbances, hallucination, euphoria, lethargy, constipation, uncoordinated movements, constipation, agitation, depression of cough reflexes, and paresthesias. Antagonists: N/V, tachycardia, hypertension, fever, and dizziness. Naltrexone is particularly hepatotoxic and can be injurious to the liver when used in high doses. Individuals with impaired liver function should be assessed carefully for signs of further damage. Monitoring: It is important to monitor for opioid withdrawal; flu-like symptoms such as muscle cramps, dilated pupils, lacrimation, rhinorrhea, yawning, sneezing, anxiety, anorexia, N/V/D, and gooseflesh. Stimulants (p. 290): Approved to treat ADHD, narcolepsy, and weight reduction. In therapeutic ranges, these drugs improve alertness, mood, attention, wakefulness, vigilance, and psychomotor performance and have an anorexiant effect. The stimulants amphetamine, dextroamphetamine, methylphenidate, and less commonly, methamphetamine (Desoxyn) are used in the treatment of ADHD and narcolepsy. Primarily, methylphenidate (Ritalin) and Adderall, a combination of dextroamphetamine (Dexedrine) and amphetamine salts are used. In addition, atomoxetine (Strattera), a norepinephrine reuptake inhibitor, is available as an alternative to stimulants for ADHD. Pharmacodynamics: The CNS stimulants are sympathomimetic amines that act as dopamine agonists and indirectly release and prevent the reuptake of dopamine, serotonin, and norepinephrine in presynaptic nerve endings. This action stimulates the cerebral cortex, brainstem, and reticular activating system and appears to stimulate the reward center of the brain that consists of the nucleus accumbens, the amygdala, and the ventral tegmentum. The dopamine and norepinephrine nerve fibers connect these regions of the brain to the prefrontal cortex to coordinate thinking, feeling, and responding to emotional stimuli. When receptors in the reward center are occupied, there is a sense of well-being; it is because of this response that these drugs have considerable potential for abuse. Precautions and Contraindications: Contraindications include arteriosclerotic and symptomatic heart disease, HTN, hyperthyroidism hypersensitivity to sympathomimetic amines, glaucoma, motortics, agitation, history of drug abuse, and during or within 14 days of use of an MAOI. Contraindicated for pregnant or lactating women (Category C). ADRs: Undesirable effects include insomnia, undesired weight loss, growth retardation in children, tachycardia, palpitations, restlessness, irritability, euphoria, headache, blurred vision, tremor, increased libido with impaired ability, HTN, and arrhythmias. Some individuals may experience a paradoxical drowsiness. Chapter 35: Chronic Migraine and Cluster Headache Chronic daily headache headaches 15 or more days a month for longer than 3 months • Chronic daily headaches (CDH) can be divided into five subtypes: o chronic tension-type headache o chronic migraine o hemicrania continua (Not in the study guide = not covered in depth) ▪ rare disorder that responds completely to indomethacin and to nothing else. Indomethacin (Indocin) 75 to 150 mg is given daily; doses up to 200 mg daily may be needed. Referral to a neurologist is recommended. o medication-overuse headache o new daily persistent headache. • Use of drugs for acute headache treatment more than 9 days a month is associated with increased risk of chronic daily headaches. • Medication-overuse is addressed later Pathophysiology: Patho of CDH is often unclear and of mixed origin. • There is a clear difference between chronic migraine and hemicrania continua (Not in the study guide = not covered). • The boundary between chronic tension-type headache and chronic migraine is less clear and may require a neurology referral for treatment. The term chronic migraine refers to CDH that starts as episodic migraine (less than 15 days a month) that transforms into a chronic pattern of greater than 15 days a month of migraine headache • It was formerly called “transformed migraine.” • The initial migraines have the pathogenesis of migraine discussed earlier. Chronic migraine is not well understood but is thought to be related to a combination of atypical pain processing, cortical hyperexcitability, neurologic inflammation, and central sensitization. • Risk factors for chronic migraine include female gender, history of head or neck injury, life stress, psychiatric disorders, and comorbid pain disorders Goals of Treatment The first goal of treatment for CDH is to break the pattern of daily headache. The patient is then stabilized on prophylactic or preventive therapy. Rational Drug Selection Chronic Migraine In most patients with chronic migraine, the daily headache cycle can be broken by using repeated doses of IV DHE (dihydroergotamine mesylate). • Approximately 70% to 80% of patients respond to DHE. o The patient is given a test dose of 0.33 mL of DHE (1 mg/mL solution) with 5 mg of metoclopramide or 10 mg of prochlorperazine (Compazine). o Followed by 0.5 mL of DHE and one of the anti-nausea medications every 6 hours for 48 to 72 hours. o This usually requires inpatient treatment. o DHE is contraindicated in coronary and peripheral vascular disease. Alternatives to DHE: • Chlorpromazine (Thorazine) • Prochlorperazine. If the patient has medication-overuse headache due to misuse of analgesics, ergots, or combination medications, the patient has to be detoxified (Discussed later) Treatment of chronic migraine may require consultation with a neurologist. Preventive pharmacotherapy can be started after the headache cycle is broken. • The patient usually responds to migraine-preventive medications such as propranolol, divalproex, or a tricyclic antidepressant. • Amitriptyline is a good choice if the patient is also depressed. • The seizure medications topiramate or valproic acid may be used. • The patient is on preventive medication until the headache days are reduced by 50%, and then an additional 3 to 4 weeks, for a total of 6 to 12 weeks. The patient should also receive alternative therapy to treat CDH. Behavioral counseling, biofeedback therapy, relaxation therapy, physical exercise, and acupuncture are all valid alternative therapies for treatment of CDH. Monitoring Monitoring of patients with CDH who are on preventive therapy requires the patient to keep a diary of headache and medication use. • Patients’ blood pressure should be monitored if they are on a beta blocker • Liver function monitored if on divalproex, as per migraine therapy monitoring. • Ongoing monitoring of headache is necessary because 31% may have recurrence of headache in spite of preventive medication. Outcome Evaluation Patients with CDH are difficult to treat. Treatment success is determined by how effective it has been in breaking the cycle of daily headaches and how effective the preventive treatment is. The patient's headache diary is key in the evaluation of the success of treatment. Patient Education Should include a discussion of information related to the overall treatment plan as well as that specific to the drug therapy, reasons for taking the drug, drugs as part of the total treatment plan, and adherence issues. Patient education information specific to treating CDH should focus on the following principles: 1. Education about the nature of the disorder, particularly that it is biological in origin, with neurochemical changes producing the headache. 2. Overuse of analgesics, leading to medication-overuse headache, must be emphasized. 3. The influence of stress, anxiety, depression, and inability to relax should be discussed, and the patient encouraged to use nonpharmacological therapies to decrease headache. CLUSTER HEADACHES: characterized by intense pain lasting for 15 minutes to 2 hours. • Occur in “clusters” of several weeks or months, with the headache subsiding for months at a time, often to recur. • The patient can experience one to three attacks a day, usually at the same time of day. They occur most frequently at night, awakening the patient from sleep. • Men are affected more than women, with onset in their late twenties. • The pain of a cluster headache is unique in that it occurs behind or around one eye, with tearing, conjunctival injection, and drooping of the eyelid common symptoms. • There may be nasal congestion, facial flushing, and sweating. The pain is so severe that the patient is unable to lie down or sit still, often pacing the floor in pain. Pathophysiology • No clear etiology for cluster headaches. • They are most likely a neuronal disorder originating in the hypothalamus. • The clockwork-like timing of cluster headaches suggests that the circadian pacemaker or biological “clock” is dysfunctional. Goals of Treatment Relieving the pain of an acute cluster headache and decreasing the length of time of the cluster are the goals of cluster headache management. Rational Drug Therapy Most patients with cluster headaches require acute and preventive therapy. The acute attacks are severe and last only a short time-intervention must be fast-acting. The patient usually requires both acute and preventive medications to manage the headache. Acute Therapy • Oxygen therapy administered via a 100% nonrebreather mask for 15 to 30 minutes often provides immediate relief of cluster headache. • Sumatriptan, administered SC, or intranasal sumatriptan or zolmitriptan may provide relief of acute cluster headaches • Intranasal lidocaine is thought to be effective in treating cluster headache. o The patient lies supine, hyperextends the head at 45 degrees, and rotates it 30 degrees to the side of the headache. o The lidocaine nasal solution is then dripped into the nostril on the affected side over 30 seconds. o The onset is approximately 5 minutes. • Ergotamine derivatives are also effective for acute cluster headaches. o Sublingual 2 mg tablets are administered at the beginning of the cluster headache. o Ergotamine suppositories or DHE intranasally or IM may also be used o Ergotamine may also be administered in a 2 mg dose given before bed if nocturnal attacks occur frequently. Preventive Therapy • Verapamil can prevent cluster headaches in some patients. • Calcium channel blockers are thought to prevent cluster headache by inhibiting vasospasm of the cerebral arteries. • Cluster headaches appear to need dosing in the high range to achieve headache reduction. • Divalproex can be effective in preventing cluster headaches. The dosing is the same as for migraine prophylaxis • Lithium appears to have some effect on cluster headaches in some patients, and a trial of lithium is warranted if the patient does not respond to other preventive medications. o The dose for cluster headache prevention is 300 mg daily to a maximum of 300 mg 3 times a day. o The patient needs careful monitoring for adverse effects, including electrocardiogram (ECG), electrolytes, thyroid function, creatinine, and CBC studies. Nonpharmacological therapies include avoidance of all alcohol during the clustering of headaches because alcohol often precipitates a headache. • Patients often are able to drink alcohol between headache clusters without adverse effects. • Tobacco, stress, anger, and vigorous physical activity should be avoided. • The patient needs to maintain a normal sleep pattern, if possible. Cluster headaches do not appear to respond to self-care measures such as massage and relaxation. Monitoring • Cluster headaches can be severely disabling, and the intense pain and loss of sleep can significantly affect the patient's quality of life. • The health-care provider needs to monitor the patient for suicidal thoughts during the headache. • The headache diary helps to monitor the effectiveness of acute and preventive medications. • A patient treated with lithium requires careful monitoring of ECG and chemistries throughout treatment. Outcome Evaluation • Cluster headaches by definition are self-limiting and will eventually stop, regardless of treatment. • The focus of care is to provide measures that shorten or prevent cluster headaches during the cluster. • Evaluation of the effectiveness of acute and preventive therapy is accomplished by self-report with a headache diary. • Modifications in pharmacological management of cluster headaches should be based on the headache diary. Patient Education Include a discussion of information related to the overall treatment plan as well as that specific to the drug therapy, reasons for taking the drug, drugs as part of the total treatment plan, and adherence issues. Patient education information specific to treating cluster headaches should focus on the following principles: 1. Educating the family about cluster headache, particularly the fact that it is a benign condition, in spite of the severe pain experienced during attacks. 2. Self-management of acute medications. The headache is usually brief, and therefore the patient must be able to self-medicate to provide relief. The pain may be gone by the time the patient can get transportation to a medical clinic. 3. Avoidance of alcohol is crucial during clusters of headaches. [Show More]
Last updated: 1 year ago
Preview 1 out of 46 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jan 29, 2022
Number of pages
46
Written in
Additional information
This document has been written for:
Uploaded
Jan 29, 2022
Downloads
0
Views
77